



API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
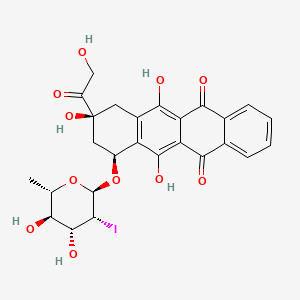

1. 2'-iodo-3'-hydroxy-4'-epi-4-demethoxydoxorubicin
1. 92689-49-1
2. 2'-iodo-3'-hydroxy-4'-epi-4-demethoxydoxorubicin
3. Snu299m83q
4. (7s,9s)-7-(((2r,3r,4r,5r,6s)-4,5-dihydroxy-3-iodo-6-methyltetrahydro-2h-pyran-2-yl)oxy)-6,9,11-trihydroxy-9-(2-hydroxyacetyl)-7,8,9,10-tetrahydrotetracene-5,12-dione
5. 5,12-naphthacenedione, 7-((2,6-dideoxy-2-iodo-alpha-l-mannopyranosyl)oxy)-7,8,9,10-tetrahydro-6,9,11-trihydroxy-9-(hydroxyacetyl)-, (7s-cis)-
6. Unii-snu299m83q
7. Ar-522
8. (7s,9s)-7-[(2r,3r,4r,5r,6s)-4,5-dihydroxy-3-iodo-6-methyloxan-2-yl]oxy-6,9,11-trihydroxy-9-(2-hydroxyacetyl)-8,10-dihydro-7h-tetracene-5,12-dione
9. Schembl19368
10. Dtxsid901027238
11. Zinc3918134
12. Db06420
13. 689a491
14. Q4767903
| Molecular Weight | 640.4 g/mol |
|---|---|
| Molecular Formula | C26H25IO11 |
| XLogP3 | 2.2 |
| Hydrogen Bond Donor Count | 6 |
| Hydrogen Bond Acceptor Count | 11 |
| Rotatable Bond Count | 4 |
| Exact Mass | 640.04416 g/mol |
| Monoisotopic Mass | 640.04416 g/mol |
| Topological Polar Surface Area | 191 Ų |
| Heavy Atom Count | 38 |
| Formal Charge | 0 |
| Complexity | 962 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 7 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Investigated for use/treatment in breast cancer and leukemia (unspecified).
Antibiotics, Antineoplastic
Chemical substances, produced by microorganisms, inhibiting or preventing the proliferation of neoplasms. (See all compounds classified as Antibiotics, Antineoplastic.)
Annamycin belongs to the anthracycline class of drugs, and has a pleiotropic mechanism of action where it targets topoisomerase II, causing strand breaks in DNA. Annamycin forms complexes with DNA by intercalation between base pairs, and it inhibits topoisomerase II activity by stabilizing the DNA-topoisomerase II complex, preventing the religation portion of the ligation-religation reaction that topoisomerase II catalyzes.


