



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
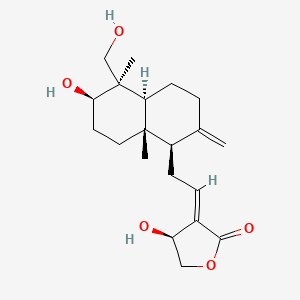

1. 17-hydro-9-dehydro-andrographolide
2. Isoandrographolide
1. 5508-58-7
2. Chebi:65408
3. (s,e)-4-hydroxy-3-(2-((1r,4as,5r,6r,8as)-6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylenedecahydronaphthalen-1-yl)ethylidene)dihydrofuran-2(3h)-one
4. Hmpl004
5. Andro
6. 410105jhgr
7. Nsc-383468
8. 3alpha,14,15,18-tetrahydroxy-5b,9bh,10a-labda-8(20),12-dien-16-oic Acid Gamma-lactone
9. (3e,4s)-3-[2-[(1r,4as,5r,6r,8as)-6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylidene-3,4,4a,6,7,8-hexahydro-1h-naphthalen-1-yl]ethylidene]-4-hydroxyoxolan-2-one
10. 3-(2-(decahydro-6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylenenaphthyl)ethylidene)dihydro-4-hydroxyfuran-2(3h)-one
11. (1r-(1-alpha(e(s)),4abeta,5alpha,6alpha,8aalpha))-3-(2-(decahydro-6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylene-1-naphthalenyl)ethylidene)dihydro-4-hydroxy-2(3h)-furanone
12. (3e,4s)-3-{2-[(1r,4as,5r,6r,8as)-6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylidene-decahydronaphthalen-1-yl]ethylidene}-4-hydroxyoxolan-2-one
13. (3e,4s)-4-hydroxy-3-{2-[(1r,4as,5r,6r,8as)-6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylidenedecahydronaphthalen-1-yl]ethylidene}dihydrofuran-2(3h)-one
14. 2(3h)-furanone, 3-[2-[(1r,4as,5r,6r,8as)-decahydro-6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylene-1-naphthalenyl]ethylidene]dihydro-4-hydroxy-, (3e,4s)-
15. Unii-410105jhgr
16. Hmpl-004
17. Nsc383468
18. Ncgc00095597-01
19. 2(3h)-furanone, 3-(2-((1r,4as,5r,6r,8as)-decahydro-6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylene-1-naphthalenyl)ethylidene)dihydro-4-hydroxy-, (3e,4s)-
20. Einecs 226-852-5
21. Nsc 383468
22. Andrographolide, 98%
23. Andrographolide [mi]
24. Dsstox_cid_25980
25. Dsstox_rid_81270
26. Dsstox_gsid_45980
27. Andrographolide [inci]
28. Bidd:er0530
29. Chembl186141
30. Gtpl9675
31. Megxp0_000978
32. Andrographolide [usp-rs]
33. Andrographolide [who-dd]
34. Dtxsid3045980
35. Schembl12056309
36. Acon1_002113
37. (3e,4s)-3-(2-((1r,4as,5r,6r,8as)-decahydro-6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylene-1-naphthalenyl)ethylidene)dihydro-4-hydroxy-2(3h)-furanone
38. 869807-57-8
39. Act03252
40. Andrographolide, Analytical Standard
41. Hy-n0191
42. Zinc3881797
43. Tox21_111508
44. Bdbm50084419
45. Mfcd07778082
46. Akos015920075
47. Ccg-208428
48. Cs-3334
49. Db05767
50. Ncgc00179817-01
51. Ncgc00179817-02
52. As-13637
53. Cas-5508-58-7
54. C20214
55. 508a587
56. A830479
57. Q-100624
58. Q4759444
59. Brd-k89282837-001-01-0
60. Andrographolide (constituent Of Andrographis) [dsc]
61. Andrographolide, United States Pharmacopeia (usp) Reference Standard
62. (3e)-3-[2-[(1r,8as)-6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylene-decalin-1-yl]ethylidene]-4-hydroxy-tetrahydrofuran-2-one;andrographolide
63. (3e,4s)-3-[2-[(1r,4as,5r,6r,8as)-6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylene-decalin-1-yl]ethylidene]-4-hydroxy-tetrahydrofuran-2-one
64. (5beta,9r,10alpha,14s)-3alpha,14,15,18-tetrahydroxylabdane-8(20),12-diene-16-oic Acid Gamma-lactone
65. (s,e)-4-hydroxy-3-(2-((1r,4as,5r,6r,8as)-6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylenedecahydronaphthalen-1-yl)ethylidene)dihydrofuran-2
66. 2(3h)-furanone, 3-(2-(decahydro-6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylene-1-naphthalenyl)ethylidene)dihydro-4-hydroxy-, (1r-(1-alpha(e(s*)),4a-beta,5-alpha,6-alpha,8a-alpha))-
67. 2(3h)-furanone,3-[2-[(1r,4as,5r,6r,8as)-decahydro-6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylene-1-naphthalenyl]ethylidene]dihydro-4-hydroxy-,(3e,4s)-
68. 3.alpha.,14,15,18-tetrahydroxy-5.beta.,9.beta.h,10.alpha.-labda-8(20),12-dien-16-oic Acid .gamma.-lactone
| Molecular Weight | 350.4 g/mol |
|---|---|
| Molecular Formula | C20H30O5 |
| XLogP3 | 2.2 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 3 |
| Exact Mass | 350.20932405 g/mol |
| Monoisotopic Mass | 350.20932405 g/mol |
| Topological Polar Surface Area | 87 Ų |
| Heavy Atom Count | 25 |
| Formal Charge | 0 |
| Complexity | 597 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 6 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Investigated for use/treatment in ulcerative colitis.
Antiprotozoal Agents
Substances that are destructive to protozoans. (See all compounds classified as Antiprotozoal Agents.)
Anti-Inflammatory Agents
Substances that reduce or suppress INFLAMMATION. (See all compounds classified as Anti-Inflammatory Agents.)
Anti-Inflammatory Agents, Non-Steroidal
Anti-inflammatory agents that are non-steroidal in nature. In addition to anti-inflammatory actions, they have analgesic, antipyretic, and platelet-inhibitory actions. They act by blocking the synthesis of prostaglandins by inhibiting cyclooxygenase, which converts arachidonic acid to cyclic endoperoxides, precursors of prostaglandins. Inhibition of prostaglandin synthesis accounts for their analgesic, antipyretic, and platelet-inhibitory actions; other mechanisms may contribute to their anti-inflammatory effects. (See all compounds classified as Anti-Inflammatory Agents, Non-Steroidal.)
Antiviral Agents
Agents used in the prophylaxis or therapy of VIRUS DISEASES. Some of the ways they may act include preventing viral replication by inhibiting viral DNA polymerase; binding to specific cell-surface receptors and inhibiting viral penetration or uncoating; inhibiting viral protein synthesis; or blocking late stages of virus assembly. (See all compounds classified as Antiviral Agents.)
Platelet Aggregation Inhibitors
Drugs or agents which antagonize or impair any mechanism leading to blood platelet aggregation, whether during the phases of activation and shape change or following the dense-granule release reaction and stimulation of the prostaglandin-thromboxane system. (See all compounds classified as Platelet Aggregation Inhibitors.)
HMPL-004 acts on multiple cellular targets in the inflammatory signal transduction pathways resulting in suppressed inflammation cytokine expression including TNF-, IL-1 and IL-6. HMPL-004 was demonstrated to inhibit TNF- and IL-1 production in cell-based assays. HMPL-004 is also able to inhibit NF-kB activation. NF-kB is a family of transcriptional factors that regulate a wide spectrum of genes critically involved in host defence and inflammation. The mechanism of action of HMPL-004 was further supported in laboratory IBD animal models. Treatment of IBD rats with HMPL-004 caused a significant drop in plasma cytokine concentrations, including TNF- and IL-1.


