
Synopsis
Synopsis
0
VMF
DRUG PRODUCT COMPOSITIONS
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
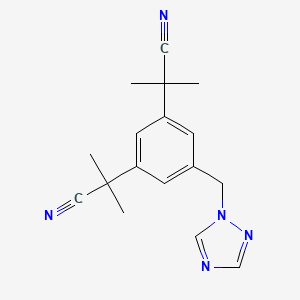

1. 2,2'-(5-(1h-1,2,4-triazol-1-ylmethyl)-1,3-phenylene)bis(2-methylpropionitrile)
2. Anastrazole
3. Arimidex
4. Ici D1033
5. Zd 1033
6. Zd-1033
7. Zd1033
8. Zeneca Zd 1033
1. 120511-73-1
2. Arimidex
3. Anastrazole
4. Anastrozol
5. Zd1033
6. 2,2'-(5-((1h-1,2,4-triazol-1-yl)methyl)-1,3-phenylene)bis(2-methylpropanenitrile)
7. Ici D1033
8. Zd-1033
9. Ici-d1033
10. 2-[3-(2-cyanopropan-2-yl)-5-(1,2,4-triazol-1-ylmethyl)phenyl]-2-methylpropanenitrile
11. Alpha,alpha,alpha',alpha'-tetramethyl-5-(1h-1,2,4-triazol-1-ylmethyl)-m-benzenediacetonitrile
12. Nsc-719344
13. Nsc-759855
14. 2z07myw1az
15. Chembl1399
16. 2,2'-[5-(1h-1,2,4-triazol-1-ylmethyl)benzene-1,3-diyl]bis(2-methylpropanenitrile)
17. Chebi:2704
18. Rvg-106400
19. Nsc719344
20. Ncgc00164619-01
21. Asiolex
22. Dsstox_cid_2607
23. 2-[3-(1-cyano-1-methylethyl)-5-(1h-1,2,4-triazol-1-ylmethyl)phenyl]-2-methylpropanenitrile
24. Dsstox_rid_76656
25. Dsstox_gsid_22607
26. Alpha1,alpha1,alpha3,alpha3-tetramethyl-5-(1h-1,2,4-triazol-1-ylmethyl)-1,3-benzenediacetonitrile
27. Zd 1033
28. Arimidex (tn)
29. Smr000466301
30. Cas-120511-73-1
31. Hsdb 7462
32. Ici-d 1033
33. Sr-01000759390
34. Unii-2z07myw1az
35. Ccris 9352
36. Anastrozole [usan:usp:inn:ban]
37. Anastrozole- Bio-x
38. Arimidex (astrazeneca)
39. Anastrozole [mi]
40. Anastrozole [inn]
41. Anastrozole [jan]
42. Anastrozole [hsdb]
43. Anastrozole [usan]
44. Schembl9726
45. Anastrozole [mart.]
46. Anastrozole [usp-rs]
47. Anastrozole [who-dd]
48. Zinc941
49. 1,3-benzenediacetonitrile, Alpha,alpha,alpha',alpha'-tetramethyl-5-(1h-1,2,4-triazol-1-ylmethyl)-
50. Mls000759396
51. Mls001424217
52. Mls006011961
53. Anastrozole (jan/usp/inn)
54. Gtpl5137
55. Dtxsid9022607
56. Bdbm10015
57. Anastrozole [orange Book]
58. Anastrozole [ep Monograph]
59. Anastrozole [usp Impurity]
60. Bcpp000401
61. Hms2052m11
62. Hms2089n10
63. Hms2235m06
64. Hms3369k05
65. Hms3394m11
66. Hms3654i18
67. Hms3715b05
68. Hms3742o21
69. Hms3866k03
70. Pharmakon1600-01502278
71. Anastrozole [usp Monograph]
72. Act03222
73. Amy42020
74. Bcp02090
75. Tox21_112238
76. Tox21_303568
77. Mfcd00866298
78. Nsc759855
79. S1188
80. Stl451008
81. Akos015894980
82. Tox21_112238_1
83. Ac-4234
84. Bcp9000301
85. Ccg-101109
86. Cs-0716
87. Db01217
88. Ks-5052
89. Nc00359
90. Nsc 719344
91. Nsc 759855
92. Sb17304
93. 2,2'-[5-(1h-1,2,4-triazol-1-ylmethyl)-1,3-phenylene]bis(2-methylpropanenitrile)
94. Ncgc00164619-02
95. Ncgc00164619-04
96. Ncgc00257356-01
97. Bt164176
98. Hy-14274
99. Sw197739-4
100. 11a731
101. C08159
102. D00960
103. Ab00639929-06
104. Ab00639929-08
105. Ab00639929-09
106. Ab00639929-10
107. Ab00639929_11
108. Ab00639929_13
109. A804526
110. Q419143
111. Sr-01000759390-4
112. Sr-01000759390-5
113. Z1522566626
114. 3-amino-3-(4-diethylamino-2-hydroxy-phenyl)-propionicacid
115. 1,.alpha.,alpha.,alpha.',.alpha.'-tetramethyl-5-(1h-1,2,4-triazol-1-ylmethyl)-
116. 2,2'-[5-(1h-1,2,4-triazol-1-yl-methyl)-1,3-phenylene]-di(2-methyl Propionitrile)
117. 2,2'-[5-(1h-1,2,4-triazol-1-ylmethyl)-1,3-phenylene]di(2-methylpropiononitrile)
118. 2,2'-[5-(1h-1,2,4-triazole-1-yl-methyl)-1,3-phenylene]di(2-methyl Propionitrile)
119. 2-[3-(2-cyano-2-propyl)-5-(1,2,4-triazol-1-ylmethyl)phenyl]-2-methylpropiononitrile
120. 2-[3-(2-cyanopropan-2-yl)-5-(1,2,4-triazol-1-ylmethyl)phenyl]-2-methyl-propanenitrile
121. .alpha.,.alpha.',.alpha.'-tetramethyl-5(1h-1,2,4-triazol-1-ylmethyl)-m-benzenediacetonitrile
122. .alpha.,.alpha.,.alpha.',.alpha.'-tetramethyl-5-(1h-1,2,4-triazol-1-ylmethyl)-m-benzenediacetonitrile
123. 1,3-benzenediacetonitrile,.alpha.,.alpha.,.alpha.',.alpha.'-tetramethyl-5-(1h-1,2,4-triazol-1-ylmethyl)-
124. 2-[3-(1-cyano-1-methylethyl)-5-[(1h-1,2,4-triazol-1-yl)methyl]phenyl]-2-methylpropanenitrile
125. Alpha,alpha,alpha',alpha'-tetramethyl-5-(1h-1,2,4-triazol-1-ylmethyl)-1,3-benzenediacetonitrile
| Molecular Weight | 293.4 g/mol |
|---|---|
| Molecular Formula | C17H19N5 |
| XLogP3 | 2.1 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 4 |
| Exact Mass | 293.16404563 g/mol |
| Monoisotopic Mass | 293.16404563 g/mol |
| Topological Polar Surface Area | 78.3 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 456 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Anastrozole |
| PubMed Health | Anastrozole (By mouth) |
| Drug Classes | Antineoplastic Agent, Endocrine-Metabolic Agent |
| Drug Label | Anastrozole tablets for oral administration contain 1 mg of anastrozole, a non-steroidal aromatase inhibitor. It is chemically described as 1,3-Benzenediacetonitrile, a, a, a', a'-tetramethyl-5-(1H-1,2,4-triazol-1-ylmethyl). Its molecular formul |
| Active Ingredient | Anastrozole |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 1mg |
| Market Status | Prescription |
| Company | Santos Biotech; Teva Pharms; Fresenius Kabi Oncol; Apotex; Accord Hlthcare; Sun Pharm Inds; Natco Pharma; Zydus Pharms Usa; Dr Reddys Labs; Sandoz; Three Rivers Pharms; Mylan; Roxane |
| 2 of 4 | |
|---|---|
| Drug Name | Arimidex |
| PubMed Health | Anastrozole (By mouth) |
| Drug Classes | Antineoplastic Agent, Endocrine-Metabolic Agent |
| Drug Label | ARIMIDEX (anastrozole) tablets for oral administration contain 1 mg of anastrozole, a non-steroidal aromatase inhibitor. It is chemically described as 1,3-Benzenediacetonitrile, a, a, a', a'-tetramethyl-5-(1H-1,2,4-triazol-1-ylmethyl). Its molec |
| Active Ingredient | Anastrozole |
| Dosage Form | Tablet |
| Route | oral; Oral |
| Strength | 1mg |
| Market Status | Prescription |
| Company | Astrazeneca |
| 3 of 4 | |
|---|---|
| Drug Name | Anastrozole |
| PubMed Health | Anastrozole (By mouth) |
| Drug Classes | Antineoplastic Agent, Endocrine-Metabolic Agent |
| Drug Label | Anastrozole tablets for oral administration contain 1 mg of anastrozole, a non-steroidal aromatase inhibitor. It is chemically described as 1,3-Benzenediacetonitrile, a, a, a', a'-tetramethyl-5-(1H-1,2,4-triazol-1-ylmethyl). Its molecular formul |
| Active Ingredient | Anastrozole |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 1mg |
| Market Status | Prescription |
| Company | Santos Biotech; Teva Pharms; Fresenius Kabi Oncol; Apotex; Accord Hlthcare; Sun Pharm Inds; Natco Pharma; Zydus Pharms Usa; Dr Reddys Labs; Sandoz; Three Rivers Pharms; Mylan; Roxane |
| 4 of 4 | |
|---|---|
| Drug Name | Arimidex |
| PubMed Health | Anastrozole (By mouth) |
| Drug Classes | Antineoplastic Agent, Endocrine-Metabolic Agent |
| Drug Label | ARIMIDEX (anastrozole) tablets for oral administration contain 1 mg of anastrozole, a non-steroidal aromatase inhibitor. It is chemically described as 1,3-Benzenediacetonitrile, a, a, a', a'-tetramethyl-5-(1H-1,2,4-triazol-1-ylmethyl). Its molec |
| Active Ingredient | Anastrozole |
| Dosage Form | Tablet |
| Route | oral; Oral |
| Strength | 1mg |
| Market Status | Prescription |
| Company | Astrazeneca |
Antineoplastic
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th Edition, Whitehouse Station, NJ: Merck and Co., Inc., 2001., p. 105
Anastrozole is indicated for the first-line treatment of postmenopausal woman with hormone receptor positive or hormone receptor unknown locally advanced or metastatic breast cancer. It is also indicated for treatment of advanced breast cancer in postmenopausal women with disease progression following tamoxifen therapy. /Included in US product label/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 151
Anastrozole is an option for the neoadjuvant treatment of hormone receptorpositive, locally advanced breast cancer in postmenopausal women. Two phase 2, randomized, double-blind clinical trials found anastrozole to be at least as effective as tamoxifen in response rates and rates of improved surgery. A phase 2, unpublished abstract reported no differences between neoadjuvant anastrozole and chemotherapy (doxorubicin and paclitaxel) in response rates, number of patients qualifying for breast-conserving surgery, and 3-year disease-free survival. An international expert panel recommends neoadjuvant endocrine therapy in postmenopausal women who would benefit from preoperative chemotherapy but are ineligible to receive it. Anastrozole was well-tolerated. /Not included in US product label/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 151
Anastrozole is not recommended for use in premenopausal women. Safety and efficacy have not been established. /Included in US product label/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 151
For more Therapeutic Uses (Complete) data for ANASTROZOLE (8 total), please visit the HSDB record page.
Among patients receiving adjuvant therapy, venous thromboembolic events occurred less frequently in patients receiving anastrozole than in those receiving tamoxifen (2 versus 4%); this included deep venous thrombosis (1 versus 2%). Ischemic cerebrovascular events also occurred less frequently in patients receiving anastrozole compared with those receiving tamoxifen (1 versus 2%). Ischemic cardiovascular disease was reported in 3% of such patients receiving anastrozole. Although angina pectoris was reported more frequently in patients receiving adjuvant therapy with anastrozole than in those receiving tamoxifen (about 2 versus 1%), the incidence of myocardial infarction was similar (0.8%).
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 927
Among patients receiving anastrozole as first-line therapy, thromboembolic disease was reported in 18 patients (4%), with 5 patients experiencing venous thrombosis (including pulmonary embolus, thrombophlebitis, and retinal vein thrombosis) and 13 patients experiencing coronary and/or cerebral thrombosis (including myocardial infarction, myocardial ischemia, angina pectoris, cerebrovascular accident, cerebral ischemia, and cerebral infarct). Despite its lack of estrogenic activity, there was no evidence of an increased incidence of myocardial infarction in patients receiving anastrozole compared with those receiving tamoxifen.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 927
Among patients receiving anastrozole as second-line therapy, thromboembolic disease was reported in 3%, and thrombophlebitis occurred in 2-5%.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 927
Among patients receiving adjuvant therapy, hot flushes (flashes) occurred less frequently in patients receiving anastrozole than in those receiving tamoxifen (35 versus 40%). Among patients receiving anastrozole as first-line or second-line therapy, hot flushes occurred in 26 or 13%, respectively.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 927
For more Drug Warnings (Complete) data for ANASTROZOLE (35 total), please visit the HSDB record page.
Anastrozole is indicated as adjunct therapy in the treatment of hormone receptor-positive early breast cancer in postmenopausal women, and as a first-line treatment for hormone receptor-positive (or hormone receptor-unknown) locally advanced or metastatic breast cancer in postmenopausal women. It may also be used in the treatment of advanced breast cancer in postmenopausal women who experience disease progression despite treatment with [tamoxifen].
Gynaecomastia, McCune-Albright syndrome, Short stature due to Growth Hormone deficiency, Testotoxicosis
Anastrozole prevents the conversion of adrenal androgens (e.g. [testosterone]) to estrogen in peripheral and tumour tissues. As the growth of many breast cancers is stimulated and/or maintained by the presence of estrogen, anastrozole helps to treat these cancers by decreasing the levels of circulating estrogens. Anastrozole has a relatively long duration of action allowing for once daily dosing - serum estradiol is reduced by approximately 70% within 24 hours of beginning therapy with 1mg once daily, and levels remain suppressed for up to 6 days following cessation of therapy. The incidence of ischemic cardiovascular events was increased during anastrozole therapy and patients with pre-existing ischemic heart disease should consider the risks and benefits of anastrozole before beginning therapy. Anastrozole has also been reported to decrease spine and hip bone mineral density (BMD), so consideration should be given to monitoring of BMD in patients receiving long-term therapy.
Antineoplastic Agents, Hormonal
Antineoplastic agents that are used to treat hormone-sensitive tumors. Hormone-sensitive tumors may be hormone-dependent, hormone-responsive, or both. A hormone-dependent tumor regresses on removal of the hormonal stimulus, by surgery or pharmacological block. Hormone-responsive tumors may regress when pharmacologic amounts of hormones are administered regardless of whether previous signs of hormone sensitivity were observed. The major hormone-responsive cancers include carcinomas of the breast, prostate, and endometrium; lymphomas; and certain leukemias. (From AMA Drug Evaluations Annual 1994, p2079) (See all compounds classified as Antineoplastic Agents, Hormonal.)
Aromatase Inhibitors
Compounds that inhibit AROMATASE in order to reduce production of estrogenic steroid hormones. (See all compounds classified as Aromatase Inhibitors.)
L02BG03
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
L - Antineoplastic and immunomodulating agents
L02 - Endocrine therapy
L02B - Hormone antagonists and related agents
L02BG - Aromatase inhibitors
L02BG03 - Anastrozole
Absorption
Anastrozole is rapidly absorbed and Tmax is typically reached within 2 hours of dosing under fasted conditions. Coadministration with food reduces the rate but not the overall extent of absorption - mean Cmax decreased by 16% and the median Tmax was extended to 5 hours when anastrozole was administered 30 minutes after ingestion of food, though this relatively minor alteration in absorption kinetics is not expected to result in clinically significant effects.
Route of Elimination
Hepatic metabolism accounts for approximately 85% of anastrozole elimination. Approximately 10% of the administered dosage is eliminated unchanged in the urine.
Volume of Distribution
The volume of distribution of anastrozole into brain tissue in mice is 3.19 mL/g. Distribution into the CNS is limited due to the activity of P-gp efflux pumps at the blood brain barrier, of which anastrozole is a substrate.
Clearance
Anastrozole's clearance is mainly via hepatic metabolism and can therefore be altered in patients with hepatic impairment - patients with stable hepatic cirrhosis exhibit an apparent oral clearance approximately 30% lower compared with patients with normal liver function. Conversely, renal impairment has a negligible effect on total drug clearance as the renal route is a relatively minor clearance pathway for anastrozole. In volunteers with severe renal impairment, renal clearance was reduced by 50% while total clearance was only reduced by approximately 10%.
Anastrozole is well absorbed into systemic circulation following oral administration. Plasma concentrations approach steady-state at about 7 days of once-daily dosing, and steady-state concentrations are approximately 3-4 times higher than concentrations achieved after a single dose of the drug. Food does not affect the extent of oral absorption of anastrozole.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 930
Within the therapeutic plasma concentration range, anastrozole is 40% bound to plasma proteins.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 930
Steady-state minimum plasma concentrations averaged 25.7 and 30.4 ng/mL, respectively, in white and Japanese postmenopausal women receiving anastrozole 1 mg daily for 16 days; serum estradiol and estrone sulfate concentrations were similar between the groups.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 930
It is not known whether anastrozole is distributed into milk in humans.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 930
For more Absorption, Distribution and Excretion (Complete) data for ANASTROZOLE (10 total), please visit the HSDB record page.
Anastrozole is primarily metabolized in the liver via oxidation and glucuronidation to a number of inactive metabolites, including hydroxyanastrozole (both free and glucuronidated) and anastrozole glucuronide. Oxidation to hydroxyanastrozole is catalyzed predominantly by CYP3A4 (as well as CYP3A5 and CYP2C8, to a lesser extent) and the direct glucuronidation of anastrozole appears to be catalyzed mainly by UGT1A4. Anastrozole may also undergo N-dealkylation to form triazole and 3,5-Bis-(2-methylpropiononitrile)-benzoic acid. Labels for anastrozole state the main metabolite found in plasma following administration is triazole, but a recent pharmacokinetic study was unable to detect any products of N-dealkylation _in vitro_.
Anastrozole is extensively metabolized in the liver. Metabolism of anastrozole occurs via N-dealkylation, hydroxylation, and glucuronidation. Three metabolites of anastrozole have been identified in human plasma and urine: triazole, a glucuronide conjugate of anastrozole, and a glucuronide conjugate of hydroxyanastrozole. Triazole, the major circulating metabolite of anastrozole, lacks pharmacologic activity, and the aromatase inhibiting activity of anastrozole results principally from the parent drug. In addition, there are several minor metabolites of anastrozole, accounting for less than 5% of an administered dose, which have not been identified.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 929
The elimination half-life of anastrozole is approximately 50 hours.
Following oral administration of anastrozole in postmenopausal women, a mean terminal elimination half-life of approximately 50 hours has been reported.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 930
Anastrazole exerts its anti-estrogenic effects via selective and competitive inhibition of the aromatase enzyme found predominantly in the adrenal glands, liver, and fatty tissues. Many breast cancers are hormone receptor-positive, meaning their growth is stimulated and/or maintained by the presence of hormones such as estrogen or progesterone. In postmenopausal women, estrogen is primarily derived from the conversion of adrenally-produced androgens into estrogens by the aromatase enzyme - by competitively inhibiting the biosynthesis of estrogen at these enzymes, anastrozole effectively suppresses circulating estrogen levels and, subsequently, the growth of hormone receptor-positive tumours.
Anastrozole is a nonsteroidal aromatase inhibitor that interferes with estradiol production in peripheral tissues. Adrenally generated androstenedione, the chief source of circulating estrogen in postmenopausal women, is converted by aromatase to estrone, which is further converted to estradiol. Growth of many breast cancer tumors containing estrogen receptors and aromatase can be promoted by estrogen.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 151
Anastrozole is a potent and selective non-steroidal aromatase inhibitor. It significantly lowers serum estradiol concentrations and has no detectable effect on formation of adrenal corticosteroids or aldosterone.
Physicians Desk Reference 60th ed, Thomson PDR, Montvale, NJ 2006., p. 666
Because estrogen acts as a growth factor for hormone-dependent breast cancer cells, anastrozole-induced reduction of serum and tumor concentrations of estrogen inhibits tumor growth and delays disease progression. Anastrozole selectively inhibits the conversion of androgens to estrogens. In postmenopausal women, ovarian secretion of estrogen declines and conversion of adrenal androgens (mainly androstenedione and testosterone) to estrone and estradiol in peripheral tissues (adipose, muscle, and liver), catalyzed by the aromatase enzyme, is the principal source of estrogens. Anastrozole inhibits the aromatase enzyme by competitively binding to the heme of the cytochrome P-450 unit of the enzyme; suppression of estrogen biosynthesis in all tissues reduces serum concentrations of circulating estrogens, including estrone, estradiol, and estrone sulfate. Anastrozole selectively inhibits synthesis of estrogens and does not affect synthesis of adrenal corticosteroid, aldosterone, or thyroid hormone. In animals, anastrozole has not been shown to possess direct progestogenic, androgenic, or estrogenic activity, but alterations in the circulating concentrations of progesterone, androgens, and estrogens have been observed.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 929

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
27
PharmaCompass offers a list of Anastrozole API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Anastrozole manufacturer or Anastrozole supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Anastrozole manufacturer or Anastrozole supplier.
PharmaCompass also assists you with knowing the Anastrozole API Price utilized in the formulation of products. Anastrozole API Price is not always fixed or binding as the Anastrozole Price is obtained through a variety of data sources. The Anastrozole Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Anastrole manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Anastrole, including repackagers and relabelers. The FDA regulates Anastrole manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Anastrole API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Anastrole manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Anastrole supplier is an individual or a company that provides Anastrole active pharmaceutical ingredient (API) or Anastrole finished formulations upon request. The Anastrole suppliers may include Anastrole API manufacturers, exporters, distributors and traders.
click here to find a list of Anastrole suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Anastrole DMF (Drug Master File) is a document detailing the whole manufacturing process of Anastrole active pharmaceutical ingredient (API) in detail. Different forms of Anastrole DMFs exist exist since differing nations have different regulations, such as Anastrole USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Anastrole DMF submitted to regulatory agencies in the US is known as a USDMF. Anastrole USDMF includes data on Anastrole's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Anastrole USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Anastrole suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Anastrole Drug Master File in Japan (Anastrole JDMF) empowers Anastrole API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Anastrole JDMF during the approval evaluation for pharmaceutical products. At the time of Anastrole JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Anastrole suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Anastrole Drug Master File in Korea (Anastrole KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Anastrole. The MFDS reviews the Anastrole KDMF as part of the drug registration process and uses the information provided in the Anastrole KDMF to evaluate the safety and efficacy of the drug.
After submitting a Anastrole KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Anastrole API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Anastrole suppliers with KDMF on PharmaCompass.
A Anastrole CEP of the European Pharmacopoeia monograph is often referred to as a Anastrole Certificate of Suitability (COS). The purpose of a Anastrole CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Anastrole EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Anastrole to their clients by showing that a Anastrole CEP has been issued for it. The manufacturer submits a Anastrole CEP (COS) as part of the market authorization procedure, and it takes on the role of a Anastrole CEP holder for the record. Additionally, the data presented in the Anastrole CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Anastrole DMF.
A Anastrole CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Anastrole CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Anastrole suppliers with CEP (COS) on PharmaCompass.
A Anastrole written confirmation (Anastrole WC) is an official document issued by a regulatory agency to a Anastrole manufacturer, verifying that the manufacturing facility of a Anastrole active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Anastrole APIs or Anastrole finished pharmaceutical products to another nation, regulatory agencies frequently require a Anastrole WC (written confirmation) as part of the regulatory process.
click here to find a list of Anastrole suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Anastrole as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Anastrole API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Anastrole as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Anastrole and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Anastrole NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Anastrole suppliers with NDC on PharmaCompass.
Anastrole Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Anastrole GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Anastrole GMP manufacturer or Anastrole GMP API supplier for your needs.
A Anastrole CoA (Certificate of Analysis) is a formal document that attests to Anastrole's compliance with Anastrole specifications and serves as a tool for batch-level quality control.
Anastrole CoA mostly includes findings from lab analyses of a specific batch. For each Anastrole CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Anastrole may be tested according to a variety of international standards, such as European Pharmacopoeia (Anastrole EP), Anastrole JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Anastrole USP).

