



API Suppliers

US DMFs Filed

CEP/COS Certifications

JDMFs Filed
0
Other Certificates
Other Suppliers
0

USA (Orange Book)
0

Europe

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
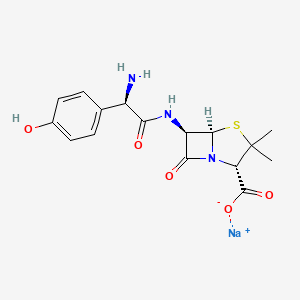

1. Actimoxi
2. Amoxicillin
3. Amoxicillin Anhydrous
4. Amoxicillin Monopotassium Salt
5. Amoxicillin Monosodium Salt
6. Amoxicillin Trihydrate
7. Amoxicillin, (r*)-isomer
8. Amoxicilline
9. Amoxil
10. Amoxycillin
11. Brl 2333
12. Brl-2333
13. Brl2333
14. Clamoxyl
15. Clamoxyl G.a.
16. Clamoxyl Parenteral
17. Hydroxyampicillin
18. Penamox
19. Polymox
20. Trimox
21. Wymox
1. 34642-77-8
2. Acuotricina
3. Ibiamox
4. Sodium Amoxicillin
5. Amoxicillin Natrium
6. Amoxicillin (sodium)
7. Amoxicillin Sodium Salt
8. Amoxicillin Sodium [usan]
9. Novabritine
10. Brl-2333ab-b
11. Amoxycillin (as Sodium)
12. Amoxicillin Sodium (amox)
13. 544y3d6myh
14. Riotapen
15. Chebi:51255
16. Amoxicillin Sodium (usan)
17. Sodium;(2s,5r,6r)-6-[[(2r)-2-amino-2-(4-hydroxyphenyl)acetyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate
18. Sodium;(2s,5r,6r)-6-[[(2r)-2-amino-2-(4-hydroxyphenyl)acetyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic Acid
19. Danoxillin
20. Amitron
21. Penamox
22. Quimiopen
23. Trifamox
24. Alfida
25. Lamoxy
26. Alfoxil Enjektabl
27. Agram Im
28. Riotapen [inj.]
29. Ibiamox [inj.]
30. Moxacin [inj.]
31. Penamox [inj.]
32. Danoxillin [inj.]
33. Lamoxy [inj.]
34. Novabritine [inj.]
35. Brl 23333ab-b
36. Unii-544y3d6myh
37. Einecs 252-124-1
38. Schembl973672
39. Chembl2105950
40. Amoxicillin Sodium [mart.]
41. Amoxicillin Sodium [who-dd]
42. Bcp12668
43. Hy-b0467
44. Sodium (2s,5r,6r)-6-((r)-2-amino-2-(4-hydroxyphenyl)acetamido)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate
45. Amoxicillin Sodium Salt [mi]
46. Mfcd08063910
47. S2565
48. Akos015951334
49. Ccg-268488
50. Amoxicillin Sodium [ep Monograph]
51. (2s-(2alpha,5alpha,6beta(s*)))-6-((amino(4-hydroxyphenyl)acetyl)amino)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo(3.2.0)heptane-2-carboxylic Acid Monosodium Salt
52. Monosodium (-)-(2s,5r,6r)-6-((r)-2-amino(4-hydroxyphenyl)acetamido)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo(3.2.0)heptane-2-carboxylate
53. D02925
54. F20449
55. Q27122484
56. Na-6-[d-a-amino-p-hydroxyphenylacetamido]penicillanic Acid
57. (2s-(2alpha,5alpha.6beta(s*)))-6-((amino(4-hydroxyphenyl)acetyl)amino)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo(3.2.0)heptane-2-carboxylic Acid Monosodium Salt
58. 4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic Acid, 6-[[(2r)-2-amino-2-(4-hydroxyphenyl)acetyl]amino]-3,3-dimethyl-7-oxo-, Sodium Salt, (2s,5r,6r)- (1:1)
59. Monosodium (-)-(2s,5r,6r)-6-((r)-2-amino-2-(p-hydroxyphenyl)acetamido)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo(3.2.0)heptane-2-carboxylate
60. Sodium (2s-(2alpha,5alpha,6beta(s*)))-6-((amino(4-hydroxyphenyl)acetyl)amino)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo(3.2.0)heptane-2-carboxylate
61. Sodium 6beta-[(2r)-2-amino-2-(4-hydroxyphenyl)acetamido]-2,2-dimethylpenam-3alpha-carboxylate
62. Sodium(2s,5r,6r)-6-((r)-2-amino-2-(4-hydroxyphenyl)acetamido)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate
| Molecular Weight | 387.4 g/mol |
|---|---|
| Molecular Formula | C16H18N3NaO5S |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 4 |
| Exact Mass | 387.08648614 g/mol |
| Monoisotopic Mass | 387.08648614 g/mol |
| Topological Polar Surface Area | 161 Ų |
| Heavy Atom Count | 26 |
| Formal Charge | 0 |
| Complexity | 596 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)


