



API Suppliers

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
Other Certificates
0
Other Suppliers
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
Uploaded Dossiers
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
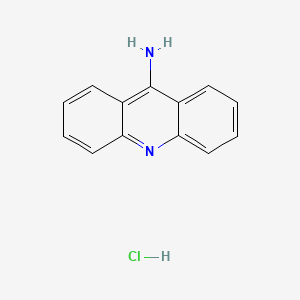

1. 9 Aminoacridine
2. 9-aminoacridine
3. Acridinamine
4. Aminacrine
5. Aminacrine Hydrochloride
6. Aminoacridine
7. Aminoacridine Hydrochloride
8. Aminopt
9. Hydrochloride, Aminacrine
10. Hydrochloride, Aminoacridine
11. Mykocert
1. Acridin-9-amine Hydrochloride
2. 134-50-9
3. Aminacrine Hydrochloride
4. Aminacrine Hcl
5. Acramine Yellow
6. Aminoacridine Hydrochloride
7. Monacrin
8. Mycosert
9. 9-aminoacridinium Chloride
10. Nsc-7571
11. 9-acridinamine Monohydrochloride
12. Monacrin Hydrochloride
13. 9-acridinamine, Monohydrochloride
14. 9-aminoacridine Monohydrochloride
15. 5-aminoacridine Hydrochloride
16. Aminacrine Hydrochloride [usan]
17. Aminoacridine Hcl
18. Or5rm3q5ql
19. Chebi:74837
20. Nsc7571
21. Nsc 7571
22. Aminoakridin
23. Quench
24. Aminacrine Hydrochloride (usan)
25. Caswell No. 033a
26. Ccris 3802
27. Hsdb 4505
28. Acridine, 9-amino-, Hydrochloride
29. Einecs 205-145-5
30. Unii-or5rm3q5ql
31. 9-aminoacridine Hcl
32. Acridine, 9-amino-, Monohydrochloride
33. Epa Pesticide Chemical Code 055503
34. Acridin-9-ylamine Hydrochloride Monohydrate
35. Ai3-16947
36. Monacrin (tn)
37. 9-acridinamine Hcl
38. Acridin-9-aminehydrochloride
39. Acridin-9-amine;hydrochloride
40. Schembl635597
41. Chembl1330022
42. Dtxsid8030473
43. 9-aminoacridinehydrochloride
44. 9-acridinamine Hydrochloride Hydrate
45. Aminacrine Hydrochloride [mi]
46. Mfcd00012663
47. 9-acridinamine, Hydrochloride (1:1)
48. Akos015903444
49. Wln: T C666 Bnj Iz & Gh
50. Aminacrine Hydrochloride [hsdb]
51. Aminacrine Hydrochloride [vandf]
52. Aminoacridine Hydrochloride [mart.]
53. Aminoacridine Hydrochloride [who-dd]
54. Db-042240
55. A0619
56. Ft-0621613
57. D02905
58. W-105829
59. Q27144949
60. Z57039678
| Molecular Weight | 230.69 g/mol |
|---|---|
| Molecular Formula | C13H11ClN2 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Exact Mass | 230.0610761 g/mol |
| Monoisotopic Mass | 230.0610761 g/mol |
| Topological Polar Surface Area | 38.9 Ų |
| Heavy Atom Count | 16 |
| Formal Charge | 0 |
| Complexity | 207 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Anti-Infective Agents, Local; Fluorescent Dyes; Indicators and Reagents; Mutagens
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
AMINACRINE HYDROCHLORIDE EXERTS GERMICICAL ACTIONS AGAINST BOTH GRAM-POSITIVE & GRAM NEGATIVE BACTERIA & AGAINST FUNGI & TRICHOMONADS. IT IS NOT INACTIVATED BY PUS, SECRETIONS, OR BODY FLUIDS. IN THE TREATMENT OF VAGINAL CANDIDIASIS, TRICHOMONIASIS, OR HAEMOPHILUS INFECTIONS ...
Gilman, A. G., L. S. Goodman, and A. Gilman. (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 6th ed. New York: Macmillan Publishing Co., Inc. 1980., p. 980
ITS PRINCIPAL USE IS IN THE TREATMENT OF INFECTIONS OF VAGINA & EXOCERVIX, SUCH AS MONILIASIS, TRICHOMONAL VAGINITIS...OR AS PROPHYLACTIC AGENT IN VARIOUS GYNECOLOGICAL PROCEDURES. IN ADDITION, IT FINDS USE IN TREATMENT OF TINEA VERSICOLOR. IT HAS ALSO BEEN USED IN TREATMENT OF MASTITIS.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 1102
EXPTL USE: EVIDENCE IS PRESENTED TO SUPPORT USE AS A SAFE & EFFECTIVE SURGICAL IRRIGANT IN DENTISTRY. AVAIL LITERATURE CONFIRMS THAT IT IS A POTENT ANTIMICROBIAL AGENT, EFFECTIVE AGAINST WIDE RANGE OF MICROORGANISMS COMMONLY FOUND IN SEPTIC WOUNDS & CAUSING MINIMAL TISSUE IRRITATION. ITS USE FOR ROUTINE ROOT CANAL IRRIGATION & AS ANTISEPTIC IN MANAGEMENT OF MAXILLOFACIA ABSCESSES IS RECOMMENDED.
SCHMITZ JP; 9-AMINOACRIDINE--ITS PRESENT STATUS AND CURRENT RECOMMENDATIONS FOR USE AS A SURGICAL AND ENDODONTIC IRRIGANT IN DENTISTRY; ORAL SURG 50(3) 273 (1980)
MEDICATION (VET): Antiseptic
Budavari, S. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. Whitehouse Station, NJ: Merck and Co., Inc., 1996., p. 72
Anti-Infective Agents, Local
Substances used on humans and other animals that destroy harmful microorganisms or inhibit their activity. They are distinguished from DISINFECTANTS, which are used on inanimate objects. (See all compounds classified as Anti-Infective Agents, Local.)
Fluorescent Dyes
Chemicals that emit light after excitation by light. The wave length of the emitted light is usually longer than that of the incident light. Fluorochromes are substances that cause fluorescence in other substances, i.e., dyes used to mark or label other compounds with fluorescent tags. (See all compounds classified as Fluorescent Dyes.)
Indicators and Reagents
Substances used for the detection, identification, analysis, etc. of chemical, biological, or pathologic processes or conditions. Indicators are substances that change in physical appearance, e.g., color, at or approaching the endpoint of a chemical titration, e.g., on the passage between acidity and alkalinity. Reagents are substances used for the detection or determination of another substance by chemical or microscopical means, especially analysis. Types of reagents are precipitants, solvents, oxidizers, reducers, fluxes, and colorimetric reagents. (From Grant and Hackh's Chemical Dictionary, 5th ed, p301, p499) (See all compounds classified as Indicators and Reagents.)
Mutagens
Chemical agents that increase the rate of genetic mutation by interfering with the function of nucleic acids. A clastogen is a specific mutagen that causes breaks in chromosomes. (See all compounds classified as Mutagens.)
IN PRESENCE OF 9-AMINOACRIDINE-HCL, BACTERIOPHAGE P22 OF SALMONELLA TYPHIMURIUM WAS KILLED ON IRRADIATION WITH VISIBLE LIGHT; NEITHER THE COMPD NOR THE LIGHT ALONE INACTIVATED THE PHAGE. THE ACRIDINE INDUCED DAMAGE IN THE PHAGES BY 4 INDEPENDENT MODES: 1) FROM SHIFT MUTAGENESIS DURING PHAGE DNA REPLICATION; 2) ACRIDINE-SENSITIZED PHYTOTOXICITY TO PHAGE PARTICLES; 3) ACRIDINE-SENSITIZED PHOTOMUTAGENESIS; AND 4) INTERFERENCE WITH VIRUS ASSEMBLY. KINETIC ANALYSIS OF FORMATION & PHOTOINACTIVATION OF COMPLEX BETWEEN 9-AMINOACRIDINE-HCL & P22 INDICATED THAT ACRIDINES BOUND TO DNA BACKBONE & INTERCALATED BETWEEN THE BASIS COULD MEDIATE LETHAL DAMAGE TO DNA-INJECTION PROTEINS.
LOECHLER E ET AL; THE USE OF SALMONELLA BACTERIOPHAGE P22 TO STUDY THE MULTIPLE MECHANISMS OF ACRIDINE-INDUCED DAMAGE; NATO CONF SER 1, 5A(IN VITRO TOXIC TEST ENVIRON AGENTS: CURR FUTURE POSSIBILITIES, PT A) 79 (1983)
INFLUENCE OF SELF-COMPLEMENTARY OLIGODEOXYNUCLEOTIDES ON CHEMICAL SHIFTS OF PROTONS OF MUTAGENIC 9-AMINOACRIDINE WAS MEASURED. UPFIELD SHIFTS INDICATIVE OF INTERCALATIVE BINDING ARE FOUND IN CASES OF DG-DC, DC-DG & DA-DT-DG-DC-DA-DT BUT NOT IN DA-DT. GEOMETRIES FOR COMPLEXES ARE COMPATIBLE WITH CHEMICAL-SHIFT DATA & X-RAY STRUCTURE OF COMPLEX BETWEEN RI5C-RG & 9-AMINOACRIDINE DETERMINED BY SAKORE ET AL CAN BE IDENTIFIED USING RECENT THEORETICAL ESTIMATES OF SHIFTS INDUCED BY NUCLEOTIDE BASES.
REUBEN J ET AL; STRUCTURE OF MUTAGEN NUCLEIC ACID COMPLEXES IN SOLUTION: PROTON CHEMICAL SHIFTS IN 9-AMINOACRIDINE COMPLEXES WITH DG-DC, DC-DG, & DA-DT-DG-DC-DA-DT; BIOCHEMISTRY 17(14) 2915 (1978)
INTERACTIONS OF 9-AMINOACRIDINE-HCL WITH IONIC CHANNELS WERE STUDIED IN INTERNALLY PERFUSED SQUID AXONS. KINETICS OF BLOCK OF SODIUM CHANNELS WITH 9-AMINOACRIDINE-HCL VARIED DEPENDING ON VOLTAGE-CLAMP PULSES & THE STATE OF GATING MACHINERY OFF SODIUM CHANNELS. IN AN AXON WITH INTACT H GATE, THE BLOCK EXHIBITED FREQUENCY- & VOLTAGE-DEPENDENT CHARACTERISTICS. IT ALSO BLOCKED POTASSIUM CHANNELS.
YEH JZ; DYNAMICS OF 9-AMINOACRIDINE BLOCK OF SODIUM CHANNELS IN SQUID AXONS; J GEN PHYSIOL 73(1) 1 (1979)
EFFECT OF SEQUENCE ON BINDING TO DNA WAS INVESTIGATED BY STUDYING ITS INTERACTION WITH DEOXYDINUCLEOSIDE PHOSPHATES OF DIFFERENT SEQUENCES USING PNMR. SIMPLEST MODEL THAT FITS THE DATA INCL 1) DIMERIZATION OF 9-AMINOACRIDINE AND 2) A MIXTURE OF 1:1 & 2:1 (DINUCLEOSIDE PHOSPHATE/9-AMINOACRIDINE) COMPLEXES. THE 1:1 COMPLEXES SEEM TO INVOLVE INTERACTION OF THE RING NITROGEN WITH THE BACKBONE PHOSPHATE & STACKING OF ONE OR BOTH CHROMOPHORES ON THE ACRIDINE; PREFERENCE IN BINDING WAS OBSERVED FOR ALTERNATING (PURINE-PYRIMIDINE OR PYRIMIDINE-PURINE) OVER NON-ALTERNATING (PURINE-PURINE) DINUCLEOSIDE PHOSPHATES. THE 2:1 COMPLEXES INVOLVE INTERCALATION OF ACRIDINE BETWEEN 2 COMPLEMENTARY DINUCLEOSIDE PHOSPHATE STRANDS WITH WEAK SEQUENCE PREFERENCES IN BINDING. STEREOCHEMISTRY OF INTERACTION DIFFERS BETWEEN NON-ALTERNATING PURINE-PURINE SEQUENCES & ALTERNATING PYRIMIDINE-PURINE OR PURINE-PYRIMIDINE SEQUENCES IN HAVING 9-AMINOACRIDINE STACKED WITH PURINES OF 1 STRAND RATHER THAN STRADDLING PURINES ON OPPOSITE STRANDS. DIFFERENCES IN STEREOCHEMISTRY COULD POSSIBLY BE DETERMINING FACTOR IN FRAMESHIFT SEQUENCE SPECIFICITY.
YOUNG PR, KALLENBACH NR; BINDING OF 9-AMINOACRIDINE TO DEOXYDINUCLEOSIDE PHOSPHATES OF DEFINED SEQUENCE: PREFERENCES AND STEREOCHEMISTRY; J MOL BIOL 145(4) 785 (1981)
For more Mechanism of Action (Complete) data for 9-AMINOACRIDINE HYDROCHLORIDE (6 total), please visit the HSDB record page.


