



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0
0

USA (Orange Book)

Europe

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
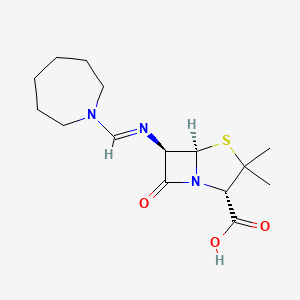

1. Amdinocillin
2. Fl 1060
3. Fl-1060
4. Fl1060
5. Penicillin Hx
1. Amdinocillin
2. 32887-01-7
3. Penicillin Hx
4. Coactin
5. Hexacillin
6. Mecillinamum
7. Selexidin
8. Mecilinamo
9. Fl 1060
10. Amdinocillin [usan]
11. Ro 10-9070
12. Fl-1060
13. Mecillinam (inn)
14. Mecillinam [inn]
15. Amdinocillin (usan)
16. Ro-10-9070
17. (2s,5r,6r)-6-(azepan-1-ylmethylideneamino)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic Acid
18. V10579p3qz
19. (2s,5r,6r)-6-[(azepan-1-ylmethylidene)amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic Acid
20. Dsstox_cid_2584
21. Dsstox_rid_76643
22. Dsstox_gsid_22584
23. Mecilinamo [inn-spanish]
24. Mecillinamum [inn-latin]
25. (2s,5r,6r)-6-{[(1e)-azepan-1-ylmethylidene]amino}-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic Acid
26. 79580-20-4
27. Smr000466361
28. Coactin (tn)
29. Ro 109070
30. Cas-32887-01-7
31. Micillinam
32. Einecs 251-277-1
33. 6-((hexahydro-1h-azepin-1-yl)methyleneamino)penicillanic Acid
34. Amdinocillin [usan:usp]
35. Unii-v10579p3qz
36. (2s,5r,6r)-6-(((hexahydro-1h-azepin-1-yl)methylene)amino)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo(3.2.0)heptane-2-carboxylic Acid
37. Ncgc00164583-01
38. Selecidin
39. Mecillinam [jan]
40. Amdinocillin [mi]
41. Chembl530
42. Mecillinam [mart.]
43. Mecillinam [who-dd]
44. Schembl34387
45. Schembl34388
46. 4-thia-1-azabicyclo(3.2.0)heptane-2-carboxylic Acid, 6-(((hexahydro-1h-azepin-1-yl)methylene)amino)-3,3-dimethyl-7-oxo-, (2s-(2alpha,5alpha,6beta))-
47. Mls000759478
48. Mls001424041
49. Mecillinam;coactin;fl 1060
50. Chembl258646
51. Dtxsid3022584
52. Amdinocillin [orange Book]
53. Hms2051e14
54. Act03258
55. Hy-a0269
56. Zinc3830206
57. Tox21_112209
58. Mfcd00056869
59. S6584
60. Zinc12469558
61. Tox21_112209_1
62. Ccg-100873
63. Db01163
64. Fs-4354
65. Nc00123
66. 6beta-[(azepan-1-ylmethylidene)amino]-2,2-dimethylpenam-3alpha-carboxylic Acid
67. Ncgc00164583-02
68. Ncgc00188988-01
69. Ac-32602
70. Cs-0017618
71. C21545
72. D02888
73. Mecillinam, Vetranal(tm), Analytical Standard
74. Ab01209740-01
75. Brd-k41051431-001-01-6
76. Q13019044
77. 6beta-{[(azepan-1-yl)methylidene]amino}-2,2-dimethylpenam-3alpha-carboxylic Acid
78. (2s,5r,6r)-6-(((e)-azepan-1-ylmethylene)amino)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic Acid
79. (2s,5r,6r)-6-(((hexahydro-1h-azepin-1-yl)methylene)amino)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo(3.2.0)heptane-2-carboxylic Acid.
80. (2s,5r,6r)-6-((e)-azepan-1-ylmethyleneamino)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic Acid
81. 4-thia-1-azabicyclo(3.2.0)heptane-2-carboxylic Acid, 6-(((hexahydro-1h-azepin-1-yl)methylene)amino)-3,3-dimethyl-7-oxo-, (2s-(2.alpha.,5.alpha.,6.beta.)0-
| Molecular Weight | 325.4 g/mol |
|---|---|
| Molecular Formula | C15H23N3O3S |
| XLogP3 | 2.1 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 3 |
| Exact Mass | 325.14601278 g/mol |
| Monoisotopic Mass | 325.14601278 g/mol |
| Topological Polar Surface Area | 98.5 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 500 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Used in the treatment of urinary tract infections caused by some strains of E. coli and klebsiella and enterobacter species. Used mainly against Gram negative organisms.
Amdinocillin is a novel, semisynthetic penicillin effective against many gram-negative bacteria. The antibacterial activity of amdinocillin is derived from its ability to bind specifically and avidly to Penicillin Binding Protein-2 (PBP 2). Amdinocillin is active alone against many gram-negative organisms. Pseudomonas and non-fermenting gram-negative bacteria, however, are usually resistant. Amdinocillin, in combination with many beta-lactams, exhibits marked synergy against many enterobacteriaceae. No such synergy can be demonstrated for gram-positive organisms or pseudomonas species. Amdinocillin is not beta-lactamase stable. Organisms which produce high levels of plasma-mediated beta-lactamase are resistant to the drug. Co-administration of probenecid results in markedly elevated plasma levels of amdinocillin and delays its excretion.
Anti-Infective Agents, Urinary
Substances capable of killing agents causing urinary tract infections or of preventing them from spreading. (See all compounds classified as Anti-Infective Agents, Urinary.)
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01C - Beta-lactam antibacterials, penicillins
J01CA - Penicillins with extended spectrum
J01CA11 - Mecillinam
Absorption
Poorly absorbed if given orally.
Approximately 1 hour in patients with normal renal function. Increases to 3 to 6 hours in anephric patients.
Amdinocillin is a stong and specific antagonist of Penicillin Binding Protein-2 (PBP 2). It is active against gram negative bacteria, preventing cell wall synthesis by inhibiting the activity of PBP2. PBP2 is a peptidoglycan elongation initiating enzyme. Peptidoglycan is a polymer of sugars and amino acids that is the main component of bacterial cell walls.


