



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
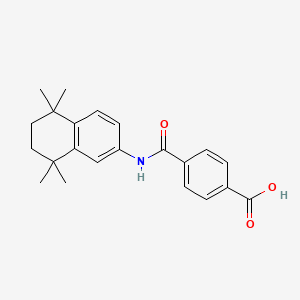

1. 4-((5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthalenyl)carbamoyl)benzoic Acid
2. Am 80
3. Am-80
4. Am80
1. 94497-51-5
2. Am 80
3. 4-((5,5,8,8-tetramethyl-5,6,7,8-tetrahydronaphthalen-2-yl)carbamoyl)benzoic Acid
4. Am-80
5. Retinobenzoic Acid
6. Am80
7. Amnoid
8. Amnolake
9. Tamibaro
10. 4-[(5,5,8,8-tetramethyl-6,7-dihydronaphthalen-2-yl)carbamoyl]benzoic Acid
11. Tos-80t
12. Inno-507
13. 4-[(5,5,8,8-tetramethyl-5,6,7,8-tetrahydronaphthalen-2-yl)carbamoyl]benzoic Acid
14. Tm-411
15. Nsc 608000
16. Nsc-608000
17. Sy-1425
18. 4-((5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthalenyl)carbamoyl)benzoic Acid
19. Chembl25202
20. Chebi:32181
21. 08v52gz3h9
22. Op-01
23. Nsc608000
24. Rr-110
25. Benzoic Acid, 4-(((5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthalenyl)amino)carbonyl)-
26. Ncgc00181111-01
27. Dsstox_cid_26853
28. Dsstox_rid_81962
29. Dsstox_gsid_46853
30. 4-[[(5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthalenyl)amino]carbonyl]benzoic Acid
31. Smr002530320
32. Cas-94497-51-5
33. Tamibarotene [inn]
34. Amnoleuk
35. Unii-08v52gz3h9
36. 2cbr
37. 4-(((5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthalenyl)amino)carbonyl)benzoic Acid
38. A80
39. Amnoid (tn)
40. Mfcd00866188
41. Tamibarotene(am-80)
42. Tetrahydrofurfurylacrylate
43. And Op-01
44. Tamibarotene (jan/inn)
45. Tamibarotene [mi]
46. Tamibarotene [jan]
47. Tamibarotene [usan:inn]
48. Tamibarotene [usan]
49. Schembl36207
50. Tamibarotene [mart.]
51. Mls003899239
52. Mls004774034
53. Mls006011150
54. Tamibarotene [who-dd]
55. Gtpl2648
56. Tos-80
57. Dtxsid5046853
58. Tamibarotene, >=98% (hplc)
59. 4-[(1,1,4,4-tetramethyltetralin-6-yl)carbamoyl]benzoic Acid
60. Hms3652h04
61. Hms3743c03
62. Zinc538415
63. Bcp07492
64. Oms-0728
65. Who 7349
66. Tox21_112725
67. Bdbm50061625
68. S4260
69. Akos015902693
70. N-(5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthyl)terephthalamic Acid
71. Tox21_112725_1
72. Ac-7049
73. Ccg-268044
74. Cs-0654
75. Db04942
76. Ncgc00181111-02
77. 121ge003
78. As-14083
79. Hy-14652
80. Nci60_004716
81. Am20060757
82. Ft-0601534
83. Sw219913-1
84. Z-208
85. D01418
86. 497t515
87. A844987
88. Q7681221
89. 4-(((5,5,8,8-tetramethyl-5,6,7,8-tetrahydro-2-naphthalenyl)amino)carbonyl)benzoic Acid
90. 4-(5,5,8,8-tetramethyl-5,6,7,8-tetrahydronaphthalen-2-ylcarbamoyl)benzoic Acid
91. 4-[[(5,6,7,8-tetrahydro-5,5,8,8-tet Ramethyl-2-naphthalenyl)amino]carbonyl]benzoic Acid
92. Terephthalic Acid Mono-5,5,8,8-tetramethyl- 5,6,7, 8-tetrahydro-2-naphthylamide
93. Terephthalic Acid Mono-5,8,8-tetramethyl- 5,6,7,8-tetrahydro-2-naphthylamide
| Molecular Weight | 351.4 g/mol |
|---|---|
| Molecular Formula | C22H25NO3 |
| XLogP3 | 5.4 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 3 |
| Exact Mass | 351.18344366 g/mol |
| Monoisotopic Mass | 351.18344366 g/mol |
| Topological Polar Surface Area | 66.4 Ų |
| Heavy Atom Count | 26 |
| Formal Charge | 0 |
| Complexity | 546 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Investigated for use/treatment in leukemia (unspecified).
Tamibarotene is a new synthetic retinoid drug recently approved for relapsed or refractory acute promyelocytic leukemia (APL) in Japan. It is a specific agonist for retinoic acid receptor alpha/beta. Compared to all-trans retinoic acid (ATRA), a natural retinoid indicated for a first-line treatment of APL, tamibarotene is chemically more stable and several times more potent as an inducer of differentiation in promyelocytic leukemia cells. In contrast to ATRA, whose plasma concentration declines considerably during daily administration, tamibarotene sustains plasma level probably due to a lower affinity for cellular retinoic acid binding protein. Furthermore, adverse side effects were milder than those of ATRA in clinical trials.
Tamibarotene is a specific agonist for retinoic acid receptor alpha/beta with possible binding to retinoid X receptors (RXR).


