



API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0
0

USA (Orange Book)
0

Europe

Canada
0

Australia
0

South Africa
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
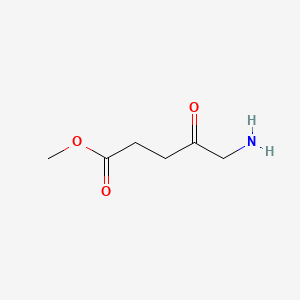

1. Maop Cpd
2. Methyl 5-aminolevulinate
3. Methyl Aminolaevulinate
4. Metvix
1. Metvix
2. 33320-16-0
3. Methyl 5-aminolevulinate
4. Methyl 5-amino-4-oxopentanoate
5. Aminolevulinic Acid Methyl Ester
6. 5-aminolevulinic Acid Methyl Ester
7. Methyl Aminolaevulinate
8. Methyl Delta-aminolevulinate
9. Pentanoic Acid, 5-amino-4-oxo-, Methyl Ester
10. D-aminolevulinicacidmethylesterhydrochloride
11. 585nm85kym
12. Chebi:724125
13. Maop Cpd
14. Ncgc00018251-03
15. Unii-585nm85kym
16. Levulinic Acid, 5-amino-, Methyl Ester
17. Methyl5-amino-4-oxopentanoate
18. Methylaminolevulinate
19. Methyl-aminolevulinate
20. Methyl-5-aminolevulinate
21. Schembl8521
22. 5-aminolavulinsauremethylester
23. Chembl1096562
24. Dtxsid3048570
25. Methyl Aminolevulinate [mi]
26. Hy-a0169
27. Zinc1909090
28. Methyl Aminolevulinate [vandf]
29. Akos006220489
30. Db00992
31. Methyl Aminolevulinate [who-dd]
32. Ncgc00018251-01
33. Ncgc00018251-02
34. Ncgc00018251-04
35. Ncgc00018251-05
36. Da-06782
37. 5-amino-4-oxo-pentanoic Acid Methyl Ester
38. Cs-0017508
39. Ft-0760150
40. D08204
41. Q619603
| Molecular Weight | 145.16 g/mol |
|---|---|
| Molecular Formula | C6H11NO3 |
| XLogP3 | -1 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 5 |
| Exact Mass | 145.07389321 g/mol |
| Monoisotopic Mass | 145.07389321 g/mol |
| Topological Polar Surface Area | 69.4 Ų |
| Heavy Atom Count | 10 |
| Formal Charge | 0 |
| Complexity | 133 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For topical use, in combination with 570 to 670 nm wavelength red light illumination, in the treatment of non-hyperkeratotic actinic keratoses of the face and scalp in immunocompetent patients when used in conjunction with lesion preparation (debridement using a sharp dermal curette).
FDA Label
After topical application of methyl aminolevulinate, porphyrins will accumulate intracellularly in the treated skin lesions. The intracellular porphyrins (including PpIX) are photoactive, fluorescing compounds and, upon light activation in the presence of oxygen, singlet oxygen is formed which causes damage to cellular compartments, in particular the mitochondria. Light activation of accumulated porphyrins leads to a photochemical reaction and thereby phototoxicity to the light-exposed target cells.
Photosensitizing Agents
Drugs that are pharmacologically inactive but when exposed to ultraviolet radiation or sunlight are converted to their active metabolite to produce a beneficial reaction affecting the diseased tissue. These compounds can be administered topically or systemically and have been used therapeutically to treat psoriasis and various types of neoplasms. (See all compounds classified as Photosensitizing Agents.)
L01XD03
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
L - Antineoplastic and immunomodulating agents
L01 - Antineoplastic agents
L01X - Other antineoplastic agents
L01XD - Sensitizers used in photodynamic/radiation therapy
L01XD03 - Methyl aminolevulinate
Absorption
In vitro, after 24 hours the mean cumulative absorption through human skin was 0.26% of the administered dose.
Photosensitization following application of methyl aminolevulinate cream occurs through the metabolic conversion of methyl aminolevulinate (prodrug) to photoactive porphyrins (PAP), which accumulates in the skin lesions to which the cream has been applied. When exposed to light of appropriate wavelength and energy, the accumulated photoactive porphyrins produce a photodynamic reaction, resulting in a cytotoxic process dependent upon the simultaneous presence of oxygen. The absorption of light results in an excited state of porphyrin molecules, and subsequent spin transfer from photoactive porphyrins to molecular oxygen generates singlet oxygen, which can further react to form superoxide and hydroxyl radicals.


