



API Suppliers

US DMFs Filed

CEP/COS Certifications

JDMFs Filed
0
Other Certificates
Other Suppliers

USA (Orange Book)

Europe

Canada

Australia

South Africa
Uploaded Dossiers
U.S. Medicaid
Annual Reports
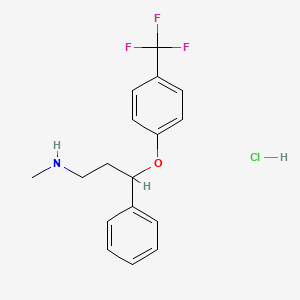

1. Fluoxetin
2. Fluoxetine
3. Lilly 110140
4. Lilly-110140
5. Lilly110140
6. N-methyl-gamma-(4-(trifluoromethyl)phenoxy)benzenepropanamine
7. Prozac
8. Sarafem
1. 56296-78-7
2. Fluoxetine Hcl
3. Prozac
4. 59333-67-4
5. Sarafem
6. Fluoxeren
7. Adofen
8. Fluctin
9. Lovan
10. Foxetin
11. Fontex
12. Fluox-puren
13. Fluoxetine (hydrochloride)
14. Fluneurin
15. Reconcile
16. Fluoxetine.hcl
17. Fluctine
18. Fluoxac
19. Fludac
20. Fluxil
21. Ly-110140
22. Fluxet
23. Ly110140
24. Ly 110140
25. Mfcd00214288
26. Selfemra
27. I9w7n6b1kj
28. Hsdb 6633
29. Fluoxetine (as Hydrochloride)
30. Mls000069361
31. Ly-110140 (free Base)
32. Chembl1201082
33. Methyl({3-phenyl-3-[4-(trifluoromethyl)phenoxy]propyl})amine Hydrochloride
34. N-methyl-3-phenyl-3-[4-(trifluoromethyl)phenoxy]propan-1-amine Hydrochloride
35. Affectine
36. Deproxin
37. Digassim
38. Flufran
39. Flunirin
40. Fluoxil
41. Flutine
42. Margrilan
43. Modipran
44. Pragmaten
45. Proctin
46. Rowexetina
47. Smr000058452
48. Erocap
49. Flunil
50. Flutin
51. Fluxen
52. Lorien
53. Neupax
54. Nopres
55. Oxedep
56. Prizma
57. Prodep
58. Sanzur
59. Sinzac
60. Zactin
61. Nuzak
62. Zepax
63. Lilly 110140
64. Prozac 20
65. Fluoxetine Hydrochloride 100 Microg/ml In Acetonitrile
66. Alzac 20
67. Profluzac
68. Prozac Weekly
69. N-methyl-3-phenyl-3-(4-(trifluoromethyl)phenoxy)propan-1-amine Hydrochloride
70. N-methyl-3-phenyl-3-[4-(trifluoromethyl)phenoxy]-1-propylamine Hydrochloride
71. N-methyl-3-[(4-trifluoromethyl)phenoxy]-3-phenylpropylamine Hydrochloride
72. Ccris 6150
73. Fluoxetinehydrochloride
74. Fluoxetine Hydrochloride [usan]
75. Sr-01000002988
76. Einecs 260-101-2
77. Lilly110140
78. Unii-i9w7n6b1kj
79. C17h18f3no.hcl
80. Framex
81. Ly-110,140 Hydrochloride
82. Fluoxetin Hcl
83. Fluoxetine Hcl
84. Fluoxetine, Hcl
85. Prozac(r)
86. Prestwick_798
87. Sarafem (tn)
88. Fluoxetine Hydrochloride [usan:usp]
89. Prozac (tn)
90. Cpd000058452
91. N-methyl-3-phenyl-3-[4-(trifluoromethyl)phenoxy]propan-1-amine;hydrochloride
92. Dsstox_cid_635
93. Opera_id_1538
94. 3-(p-trifluoromethylphenoxy)-n-methyl-3-phenylpropylamine Hydrochloride
95. (+-)-methyl-gamma-(4-(trifluoromethyl)phenoxy)benzenepropanamine Hydrochloride
96. (+-)-n-methyl-3-phenyl-3-(4-(trifluoromethyl)phenoxy)propylamine Hydrochloride
97. (+/-)-n-methyl-&gamma
98. Dsstox_rid_75706
99. Dsstox_gsid_20635
100. Schembl33384
101. Mls001076338
102. Mls002222264
103. Spectrum1504173
104. Fluoxetine Hydrochloride, Solid
105. Dtxsid7020635
106. Hy-b0102a
107. Chebi:145458
108. Hms1569h03
109. Hms1922f07
110. Pharmakon1600-01504173
111. Bcp19962
112. Npl-2008
113. Fluoxetine Hydrochloride (jan/usp)
114. Tox21_200182
115. Tox21_500558
116. Ccg-39084
117. Fluoxetine Hydrochloride [mi]
118. Methyl(3-phenyl-3-(4-(trifluoromethyl)phenoxy)propyl)ammonium Chloride
119. Nsc714457
120. Nsc758685
121. Prozac; Ly-110,140 Hydrochloride
122. S1333
123. Fluoxetine Hydrochloride [jan]
124. Akos015894939
125. Propylamine, N-methyl-3-phenyl-3-(p-trifluoromethylphenoxy)-, Hydrochloride
126. Ab04860
127. Ac-1697
128. Cs-1838
129. Fluoxetine Hydrochloride [hsdb]
130. Ks-1061
131. Lp00558
132. Nc00715
133. Nsc-714457
134. Benzenepropanamine, N-methyl-gamma-(4-(trifluoromethyl)phenoxy)-, Hydrochloride
135. Fluoxetine Hydrochloride [vandf]
136. N-methyl-3-phenyl-3-{[4-(trifluoromethyl)phenyl]oxy}propan-1-amine Hydrochloride
137. Fluoxetine Hydrochloride [usp-rs]
138. Fluoxetine Hydrochloride [who-dd]
139. Ncgc00093943-01
140. Ncgc00093943-02
141. Ncgc00093943-03
142. Ncgc00093943-04
143. Ncgc00093943-05
144. Ncgc00257736-01
145. Ncgc00261243-01
146. Bf163653
147. Sy035644
148. Cas-56296-78-7
149. Eu-0100558
150. F-132
151. F0750
152. Fluoxetine Hydrochloride [green Book]
153. Ft-0626490
154. Ft-0652216
155. Ft-0668749
156. Ft-0668750
157. Ft-0668751
158. Sw196934-4
159. Fluoxetine Hydrochloride [orange Book]
160. D00823
161. Fluoxetine Hydrochloride [ep Monograph]
162. Fluoxetine Hydrochloride [usp Impurity]
163. Fluoxetine Hydrochloride [usp Monograph]
164. Symbyax Component Fluoxetine Hydrochloride
165. 910f893
166. A830990
167. Fluoxetine Hydrochloride 100 Microg/ml In Methanol
168. Fluoxetine Hydrochloride Component Of Symbyax
169. J-521372
170. Sr-01000002988-2
171. Sr-01000002988-4
172. Sr-01000002988-9
173. Q27280620
174. Z1741982921
175. Fluoxetine Hydrochloride, Vetranal(tm), Analytical Standard
176. Fluoxetine Hydrochloride 1.0 Mg/ml In Methanol (as Free Base)
177. Fluoxetine Hydrochloride, British Pharmacopoeia (bp) Reference Standard
178. Fluoxetine Hydrochloride, European Pharmacopoeia (ep) Reference Standard
179. Methyl-[(3r)-3-phenyl-3-[4-(trifluoromethyl)phenoxy]propyl]ammonium
180. Methyl-[3-phenyl-3-(4-trifluoromethyl-phenoxy)-propyl]-amine Hydrochloride
181. N-methyl-3-phenyl-3-(p-trifluoromethylphenoxy)-propylaminhydrochloride
182. N-methyl-3-phenyl-3-[4-(trifluoromethyl)phenoxy]propylamine Hydrochloride
183. Fluoxetine Hydrochloride, Pharmaceutical Secondary Standard; Certified Reference Material
184. Fluoxetine Hydrochloride, United States Pharmacopeia (usp) Reference Standard
185. N-methyl-3-phenyl-3-(4-(trifluoromethyl)phenoxy)propan-1-amine Hydrochloride1
186. N-methyl-3-phenyl-3-(4-(trifluoromethyl)phenoxy)propan-1-aminehydrochloride1
187. N-methyl-3-phenyl-3-[4-(trifluoromethyl)phenoxy]-1-propanamine Hydrochloride (1:1)
188. N-methyl-3-phenyl-3-[4-(trifluoromethyl)phenoxy]propan-1-amine;hydrochloride.
189. (+/-)-n-methyl-3-phenyl-3-((.alpha.,.alpha.,.alpha.-trifluoro-p-tolyl)oxy)propylamine, Hydrochloride
190. Benzenepropanamine, N-methyl-.gamma.-(4-(trifluoromethyl)phenoxy)-, Hydrochloride (1:1)
191. Benzenepropanamine, N-methyl-.gamma.-(4-(trifluoromethyl)phenoxy)-, Hydrochloride, (+/-)-
192. Fluoxetine Hydrochloride Solution, 1.0 Mg/ml In Methanol (as Free Base), Ampule Of 1 Ml, Certified Reference Material
| Molecular Weight | 345.8 g/mol |
|---|---|
| Molecular Formula | C17H19ClF3NO |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 6 |
| Exact Mass | 345.1107264 g/mol |
| Monoisotopic Mass | 345.1107264 g/mol |
| Topological Polar Surface Area | 21.3 Ų |
| Heavy Atom Count | 23 |
| Formal Charge | 0 |
| Complexity | 308 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 8 | |
|---|---|
| Drug Name | Fluoxetine hydrochloride |
| Active Ingredient | Fluoxetine hydrochloride |
| Dosage Form | Tablet; Capsule; Capsule, delayed rel pellets; Solution |
| Route | Oral |
| Strength | eq 20mg base; eq 40mg base; eq 90mg base; eq 20mg base/5ml; eq 10mg base; eq 60mg base |
| Market Status | Prescription |
| Company | Ranbaxy; Mylan Pharms; Wockhardt; Silarx; Ani Pharms; Edgemont Pharms; Teva; Pharm Assoc; Alembic Pharms; Aurobindo Pharma; Teva Pharms Usa; Mallinckrodt; Sandoz; Par Pharm; Ivax Sub Teva Pharms; Lannett; Aurobindo Pharm; Landela Pharm; Dr Reddys Labs; My |
| 2 of 8 | |
|---|---|
| Drug Name | Prozac |
| PubMed Health | Fluoxetine (By mouth) |
| Drug Classes | Antidepressant, Central Nervous System Agent |
| Drug Label | SARAFEM (fluoxetine hydrochloride tablets) is a selective serotonin reuptake inhibitor(SSRI) for oral administration. It is designated ()-N-methyl-3-phenyl-3-[(,,-trifluoro-p-tolyl)oxy]propylamine hydrochloride and has the empirical formula... |
| Active Ingredient | Fluoxetine hydrochloride |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | eq 20mg base; eq 40mg base; eq 10mg base |
| Market Status | Prescription |
| Company | Eli Lilly And |
| 3 of 8 | |
|---|---|
| Drug Name | Sarafem |
| Drug Label | Fluoxetine hydrochloride is a psychotropic drug for oral administration. It is also marketed for the treatment of premenstrual dysphoric disorder (Sarafem, fluoxetine hydrochloride). It is designated ()-N-methyl-3-phenyl-3-[(a,a,a-trifluoro-p-tol... |
| Active Ingredient | Fluoxetine hydrochloride |
| Dosage Form | Tablet; Capsule |
| Route | Oral |
| Strength | eq 20mg base; eq 15mg base; eq 10mg base |
| Market Status | Prescription |
| Company | Warner Chilcott; Eli Lilly And |
| 4 of 8 | |
|---|---|
| Drug Name | Selfemra |
| PubMed Health | Fluoxetine (By mouth) |
| Drug Classes | Antidepressant, Central Nervous System Agent |
| Drug Label | DESCRIPTIONProzac (fluoxetine capsules, USP and fluoxetine oral solution, USP) is a psychotropic drug for oral administration. It is also marketed for the treatment of premenstrual dysphoric disorder (Sarafem, fluoxetine hydrochloride). It is des... |
| Active Ingredient | Fluoxetine hydrochloride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 20mg base; eq 15mg base; eq 10mg base |
| Market Status | Prescription |
| Company | Teva Pharms Usa |
| 5 of 8 | |
|---|---|
| Drug Name | Fluoxetine hydrochloride |
| Active Ingredient | Fluoxetine hydrochloride |
| Dosage Form | Tablet; Capsule; Capsule, delayed rel pellets; Solution |
| Route | Oral |
| Strength | eq 20mg base; eq 40mg base; eq 90mg base; eq 20mg base/5ml; eq 10mg base; eq 60mg base |
| Market Status | Prescription |
| Company | Ranbaxy; Mylan Pharms; Wockhardt; Silarx; Ani Pharms; Edgemont Pharms; Teva; Pharm Assoc; Alembic Pharms; Aurobindo Pharma; Teva Pharms Usa; Mallinckrodt; Sandoz; Par Pharm; Ivax Sub Teva Pharms; Lannett; Aurobindo Pharm; Landela Pharm; Dr Reddys Labs; My |
| 6 of 8 | |
|---|---|
| Drug Name | Prozac |
| PubMed Health | Fluoxetine (By mouth) |
| Drug Classes | Antidepressant, Central Nervous System Agent |
| Drug Label | SARAFEM (fluoxetine hydrochloride tablets) is a selective serotonin reuptake inhibitor(SSRI) for oral administration. It is designated ()-N-methyl-3-phenyl-3-[(,,-trifluoro-p-tolyl)oxy]propylamine hydrochloride and has the empirical formula... |
| Active Ingredient | Fluoxetine hydrochloride |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | eq 20mg base; eq 40mg base; eq 10mg base |
| Market Status | Prescription |
| Company | Eli Lilly And |
| 7 of 8 | |
|---|---|
| Drug Name | Sarafem |
| Drug Label | Fluoxetine hydrochloride is a psychotropic drug for oral administration. It is also marketed for the treatment of premenstrual dysphoric disorder (Sarafem, fluoxetine hydrochloride). It is designated ()-N-methyl-3-phenyl-3-[(a,a,a-trifluoro-p-tol... |
| Active Ingredient | Fluoxetine hydrochloride |
| Dosage Form | Tablet; Capsule |
| Route | Oral |
| Strength | eq 20mg base; eq 15mg base; eq 10mg base |
| Market Status | Prescription |
| Company | Warner Chilcott; Eli Lilly And |
| 8 of 8 | |
|---|---|
| Drug Name | Selfemra |
| PubMed Health | Fluoxetine (By mouth) |
| Drug Classes | Antidepressant, Central Nervous System Agent |
| Drug Label | DESCRIPTIONProzac (fluoxetine capsules, USP and fluoxetine oral solution, USP) is a psychotropic drug for oral administration. It is also marketed for the treatment of premenstrual dysphoric disorder (Sarafem, fluoxetine hydrochloride). It is des... |
| Active Ingredient | Fluoxetine hydrochloride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 20mg base; eq 15mg base; eq 10mg base |
| Market Status | Prescription |
| Company | Teva Pharms Usa |
Antidepressive Agents, Second-Generation; Serotonin Uptake Inhibitors
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Fluoxetine is indicated for the treatment of obsessions and compulsions in patients with obsessive-compulsive disorder. /Included in US product labeling/
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 23rd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2003. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 1359
Fluoxetine is used to relieve the symptoms of premenstrual dysphoric disorder (PMDD). PMDD was formerly known as late luteal phase dysphoric disorder (LLPDD) and is distinguishable from the cyclic changes in mood commonly known as premenstrual syndrome (PMS) by its greater severity of symptoms. (Evidence rating: B-1) /Included in US product labeling/
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 23rd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2003. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 1359
Fluoxetine is indicated for the treatment of major depressive disorder. Treatment of acute depressive episodes typically requires 6 to 12 months of antidepressant therapy. Patients with recurrent or chronic depression may require long-term treatment. Fluoxetine has shown effective maintenance of antidepressant response for up to 50 weeks of treatment in a placebo-controlled trial. /Included in US product labeling/
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 23rd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2003. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 1359
For more Therapeutic Uses (Complete) data for FLUOXETINE HYDROCHLORIDE (10 total), please visit the HSDB record page.
Pregnancy risk category: C /RISK CANNOT BE RULED OUT. Adequate, well controlled human studies are lacking, and animal studies have shown risk to the fetus or are lacking as well. There is a chance of fetal harm if the drug is given during pregnancy; but the potential benefits may outweigh the potential risk./
Physicians Desk Reference. 58th ed. Thomson PDR. Montvale, NJ 2004., p. 1843
Fluoxetine potentially may alter blood glucose concentrations. Hypoglycemia has occurred in less than 1% of patients receiving fluoxetine and hypoglycemic reaction has occurred rarely. In addition, hyperglycemia has developed following discontinuance of the drug. Therefore, the possibility that insulin and/or oral sulfonylurea antidiabetic agent dosage adjustments may be necessary when fluoxetine therapy is initiated or discontinued in patients with diabetes mellitus should be considered.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 2206
The most frequent adverse effect associated with fluoxetine therapy is nausea, which occurs in about 21% of patients. Nausea generally is mild, occurs early in therapy, and usually subsides after a few weeks of continued therapy with the drug. ... Diarrhea occurs in about 12%, anorexia in about 9%, and dyspepsia in about 6% of patients receiving the drug; limited evidence suggests that the incidence of anorexia may be dose-related. Other adverse GI effects associated with fluoxetine therapy include abdominal pain and change in taste perception, which occur in approximately 3 and 2% of patients, respectively; taste loss has been reported rarely. Vomiting, melena, and flatulence reportedly occur in about 2% and gastroenteritis in about 1% of patients receiving the drug. Increased appetite has been reported in more than 1% of patients receiving fluoxetine, but has not been definitely attributed to the drug. Other adverse GI effects, including aphthous stomatitis, dysphagia, eructation, esophagitis, gastritis, gingivitis, glossitis, melena, stomatitis, and thirst, have been reported in less than 1% of fluoxetine-treated patients; however, a causal relationship to the drug has not been established. Bloody diarrhea, colitis, duodenal or gastric ulcer, enteritis, fecal incontinence, hematemesis, hyperchlorhydria, increased salivation, mouth ulceration, salivary gland enlargement, tongue discoloration, and tongue edema have occurred rarely, but have not been definitely attributed to fluoxetine.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 2206
Because of the possibility of suicide in depressed patients, close supervision of high risk patients is recommended during initial fluoxetine therapy. To reduce the risk of overdosage, the drug should be prescribed in the smallest quantity consistent with good patient management. Suicidal ideation may persist until substantial remission of the depressive disorder occurs.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 2207
For more Drug Warnings (Complete) data for FLUOXETINE HYDROCHLORIDE (14 total), please visit the HSDB record page.
As an aid in the treatment of separation-related disorders in dogs manifested by destruction and inappropriate behaviours (vocalisation and inappropriate defecation and / or urination) and only in combination with behavioural-modification techniques.
Cytochrome P-450 CYP2D6 Inhibitors
Drugs and compounds which inhibit or antagonize the biosynthesis or actions of CYTOCHROME P-450 CYP2D6. (See all compounds classified as Cytochrome P-450 CYP2D6 Inhibitors.)
Selective Serotonin Reuptake Inhibitors
Compounds that specifically inhibit the reuptake of serotonin in the brain. (See all compounds classified as Selective Serotonin Reuptake Inhibitors.)
Antidepressive Agents, Second-Generation
A structurally and mechanistically diverse group of drugs that are not tricyclics or monoamine oxidase inhibitors. The most clinically important appear to act selectively on serotonergic systems, especially by inhibiting serotonin reuptake. (See all compounds classified as Antidepressive Agents, Second-Generation.)
QN06AB03
Fluoxetine hydrochloride appears to be well absorbed from the GI tract following oral administration. The oral bioavailability of fluoxetine in humans has not been fully elucidated to date, but at least 60-80% of an oral dose appears to be absorbed. However, the relative proportion of an oral dose reaching systemic circulation unchanged currently is not known. Limited data from animals suggest that the drug may undergo first-pass metabolism and extraction in the liver and/or lung following oral administration. In these animals (beagles), approximately 72% of an oral dose reached systemic circulation unchanged. Food appears to cause a slight decrease in the rate, but not the extent of absorption of fluoxetine in humans.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 2214
Distribution of fluoxetine and its metabolites into human body tissues and fluids has not been fully characterized. Limited pharmacokinetic data obtained during long term administration of fluoxetine to animals suggest that the drug and some of its metabolites, including norfluoxetine, are widely distributed in body tissues, with highest concentrations occurring in the lungs and liver. The drug crosses the blood-brain barrier in humans and animals. In animals, fluoxetine: norfluoxetine ratios reportedly were similar in the cerebral cortex, corpus striatum, hippocampus, hypothalamus, brain stem, and cerebellum 1 hr after administration of single dose of the drug.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 2214
In order to confirm embryonic/fetal exposure to fluoxetine and/or metabolites, dissection and whole-body autoradiographic techniques were utilized to determine the placental transfer and fetal distribution in 12 and 18 day pregnant Wistar rats 1, 4, 8, and 24 hr following a single oral 12.5 mg/kg dose of (14)C fluoxetine. On gestation Days 12 (organogenesis) and 18 (postorganogenesis), peak concentrations of radiocarbon occurred 4-8 hr after dose administration in the placenta, embryo/fetus, amniotic fluid, and maternal kidney, brain, and lung, and declined slightly at 24 hr postdose. Maternal lung contained the highest tissue concentration of radiocarbon at all time points. Placenta and maternal brain, kidney, and liver contained moderate levels of radioactivity, while embryonic/fetal tissue, amniotic fluid, and maternal plasma contained low levels of radioactivity. Mean fetal concentrations of radiocarbon at 4, 8, and 24 hr on gestation Day 18 were higher than mean embryonic concentrations on Day 12 of gestation. Analytical characterization of radioactivity indicated that combined fluoxetine and norfluoxetine concentrations accounted for 63-80% of the total radiocarbon concentrations in embryonic/fetal tissue. Results indicated that embryonic/fetal and maternal tissue levels of fluoxetine were greatest at early time points and declined with time, while norfluoxetine tissue levels were highest at the 24 hr time point. Whole-body autoradiographic techniques demonstrated that radioactivity associated with (14)C fluoxetine and/or its metabolites traversed the placenta and distributed throughout the 18 day fetus 4 hr following dose administration. Visual and quantitative evaluations of the autoradiograms indicated that the highest fetal concentrations of radiocarbon were associated with brain and thymus. Results from these studies indicate that fluoxetine and norfluoxetine traverse the placenta and distribute within the embryo/fetus during the periods of organogenesis and postorganogenesis and confirm embryonic/fetal exposure of parent and metabolite in previous negative rat teratology and reproductive studies.
PMID:2785300 Pohland RC et al; Toxicol Appl Pharmacol 98 (2): 198-205 (1989)
Elimination: Renal: 80% excreted in the urine (11.6% fluoxetine, 7.4% fluoxetine glucuronide, 6.8% norfluoxetine, 8.2% norfluoxetine glucuronide, >20% hippuric acid, 46% other); Biliary: Approximately 15% in the feces; In dialysis--Not dialyzable because of high protein binding and large volume of distribution.
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 23rd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2003. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 1359
For more Absorption, Distribution and Excretion (Complete) data for FLUOXETINE HYDROCHLORIDE (6 total), please visit the HSDB record page.
The present study was designed to define the kinetic behavior of fluoxetine N-demethylation in human liver microsomes and to identify the isoforms of cytochrome p450 (CYP) involved in this metabolic pathway. The kinetics of Ne formation of norfluoxetine was determined in human liver microsomes from six genotyped CYP2C19 extensive metabolizers (EM). The correlation studies between the fluoxetine N-demethylase activity and various CYP enzyme activities were performed. Selective inhibitors or chemical probes of various cytochrome P-450 isoforms were also employed. The kinetics of norfluoxetine formation in all liver microsomes were fitted by a single-enzyme Michaelis-Menten equation (mean Km=32 umol/L +/- 7 umol/L). Significant correlations were found between N-demethylation of fluoxetine at both 25 umol/L and 100 umol/L and 3-hydroxylation of tolbutamide at 250 micromol/L (r1=0.821, P1=0.001; r2=0.668, P2=0.013), respectively, and S-mephenytoin 4'-hydroxylase activity (r=0.717, P=0.006) at high substrate concentration of 100 umol/L. S-mephenytoin (SMP) (a CYP2C19 substrate) at high concentration and sulfaphenazole (SUL) (a selective inhibitor of CYP2C9) substantially inhibited norfluoxetine formation. The reaction was minimally inhibited by coincubation with chemical probe, inhibitor of CYP3A4 (triacetyloleandomycin, TAO). The inhibition of fluoxetine N-demethylation at high substrate concentration (100 umol/L) was greater in PM livers than in EM livers (73 % vs 45 %, P < 0.01) when the microsomes were precoincubated with SUL plus TAO. Cytochrome p450 CYP2C9 is likely to be a major CYP isoform catalyzing fluoxetine N-demethylation in human liver microsomes at a substrate concentration close to the therapeutic level, while polymorphic CYP2C19 may play a more important role in this metabolic pathway at high substrate concentration.
PMID:11730569 Liu ZQ et al; Acta Pharmacol Sin 22 (1): 85-90 (2001)
The exact metabolic fate of fluoxetine has not been fully elucidated. The drug appears to be metabolized extensively, probably in the liver, to norfluoxetine and several other metabolites. Norfluoxetine (desmethylfluoxetine) the principal metabolite, is formed by N-demethylation of fluoxetine, which may be under polygenic control. The potency and selectivity of norfluoxetine's serotonin-reuptake inhibiting activity appear to be similar to those of the parent drug. Both fluoxetine and norfluoxetine undergo conjugation with glucuronic acid in the liver, and limited evidence from animals suggests that both the parent drug and its principal metabolite also undergo O-dealkylation to form p-trifluoromethylphenol, which subsequently appears to be metabolized to hippuric acid.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 2214
The half-life of fluoxetine reportedly is prolonged (to approximately 4-5 days) after administration of multiple versus single doses, suggesting a nonlinear pattern of drug accumulation during long-term administration.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 2214
Following a single oral dose of fluoxetine in healthy adults, the elimination half-life of fluoxetine reportedly averages approximately 2-3 days (range: 1-9 days) and that of norfluoxetine averages about 7-9 days (range: 3-15 days).
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 2214
The mean half-life /for fluoxetine/ was 6.6 vs 2.2 days ... for patients with cirrhosis vs normal volunteers.
PMID:3262026 Schenker S et al; Clin Pharmacol Ther 44 (3): 353-9 (1988)
The precise mechanism of antidepressant action of fluoxetine is unclear, but the drug has been shown to selectively inhibit the reuptake of serotonin (5-HT) at the presynaptic neuronal membrane. Fluoxetine-induced inhibition of serotonin reuptake causes increased synaptic concentrations of serotonin in the CNS, resulting in numerous functional changes associated with enhanced serotonergic neurotransmission.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 2212
Monoamine oxidase-B has been determined to be the enzyme responsible for the conversion of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine into its toxic metabolite 1-methyl-4-phenylpyridine ion. Since this enzyme has been localized primarily in astrocytes and serotonergic neurons, it would appear that 1-methyl-4-phenylpyridine ion is being produced outside the dopaminergic neurons. To investigate this possibility, the administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine was preceded by systemically administered fluoxetine. In keeping with its demonstrated ability to inhibit uptake into serotonergic neurons and serotonin uptake into astrocytes, fluoxetine pretreatment resulted in a significant attenuation of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine induced depletions of striatal dopamine and serotonin concentration. These results support the extra-dopaminergic production of 1-methyl-4-phenylpyridine ion.
PMID:3258013 Brooks WJ et al; J Neural Transm 71 (2): 85-90 (1988)
Fluoxetine is a potent and selective inhibitor of the neuronal serotonin-uptake carrier and is a clinically effective antidepressant. Although fluoxetine is used therapeutically as the racemate, there appears to be a small but demonstrable stereospecificity associated with its interactions with the serotonin-uptake carrier. The goals of this study were to determine the absolute configurations of the enantiomers of fluoxetine and to examine whether the actions of fluoxetine in behavioral tests were enantiospecific. (S)-Fluoxetine was synthesized from (S)-(-)-3-chloro-1-phenylpropanol by sequential reaction with sodium iodide, methylamine, sodium hydride, and 4-fluorobenzotrifluoride. (S)-Fluoxetine is dextrorotatory (+1.60) in methanol, but is levorotatory (-10.85) in water. Fluoxetine enantiomers were derivatized with (R)-1-(1-naphthyl)ethyl isocyanate, and the resulting ureas were assayed by 1H NMR or HPLC to determine optical purities of the fluoxetine samples. Both enantiomers antagonized writhing in mice; following sc administration of (R)- and (S)-fluoxetine, ED50 values were 15.3 and 25.7 mg/kg, respectively. Moreover, both enantiomers potentiated a subthreshold analgesic dose (0.25 mg/kg) of morphine, and ED50 values were 3.6 and 5.7 mg/kg, respectively. Following ip administration to mice, the two stereoisomers antagonized p-chloroamphetamine-induced depletion of whole brain serotonin concentrations. ED50 values for (S)- and (R)-fluoxetine were 1.2 and 2.1 mg/kg, respectively. The two enantiomers decreased palatability-induced ingestion following ip administration to rats; (R)- and (S)-fluoxetine reduced saccharin-induced drinking with ED50 values of 6.1 and 4.9 mg/kg, respectively. Thus, in all biochemical and pharmacological studies to date, the eudismic ratio for the fluoxetine enantiomers is near unity.
PMID:3260286 Robertson DW et al; J Med Chem 31 (7): 1412-7 (1988)








