



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
Other Certificates
Other Suppliers
0

USA (Orange Book)

Europe

Canada

Australia
0

South Africa
Uploaded Dossiers
U.S. Medicaid
0
Annual Reports
0
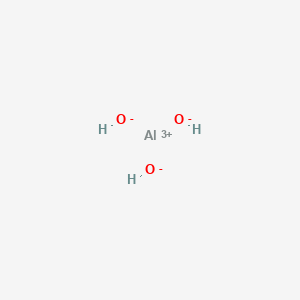

1. Dried Aluminum Hydroxide Gel
2. Aluminum;trihydroxide
3. Aluminium Hydroxide Gel, Dried
4. Dried Aluminium Hydroxide
5. Aluminum Hydroxide, Dried
6. Aluminium Hydroxide, Dried
7. Aluminum Hydroxide Gel, Dried
8. Nsc-664400
9. Aluminum Hydrate
10. Aluminum Hyroxide
11. Aluminium Trihydroxide
12. Hydroxyde D' Aluminium
13. Algeldrate Anhydrous
14. Aluminum (as Hydroxide)
15. Aluminum Hydroxide [ii]
16. Aluminum Hydroxide [mi]
17. Chembl1200706
18. Dtxsid2036405
19. Niosh/bd0708000
20. Aluminum Hydroxide [inci]
21. Di-mu-hydroxytetrahydroxydialuminum
22. Aluminum Hydroxide [vandf]
23. Aluminium Hydroxide[who-ip]
24. Aluminum Hydroxide [mart.]
25. Aluminum Hydroxide Gel,dried
26. Aluminium Hydroxide Dried Gel
27. Af-260
28. Aluminium Hydroxide [who-dd]
29. Akos015904617
30. Aluminum, Di-mu-hydroxytetrahydroxydi-
31. Db06723
32. Aluminum Hydroxide [orange Book]
33. Aluminii Hydroxidum[who-ip Latin]
34. Aluminum (as Hydroxide) [vandf]
35. Aluminum Hydroxide [usp Impurity]
36. Aluminum Hydroxide, Dried [hsdb]
37. As04 Component Aluminum Hydroxide
38. Aluminum Hydroxide Gel, Dried [ii]
39. Foamcoat Component Aluminum Hydroxide
40. Foamicon Component Aluminum Hydroxide
41. Gaviscon Component Aluminum Hydroxide
42. Aluminum Hydroxide Gel,dried [vandf]
43. Bd07080000
44. Dried Aluminum Hydroxide Gel [usp-rs]
45. Aluminum Hydroxide Component Of Foamcoat
46. Aluminum Hydroxide Component Of Foamicon
47. Aluminum Hydroxide Component Of Gaviscon
48. Aluminium Hydroxide Gel, Dried [who-dd]
49. Aluminum Hydroxide, Dried [usp Impurity]
50. Q407125
51. J-014205
52. 8012-63-3
| Molecular Weight | 78.004 g/mol |
|---|---|
| Molecular Formula | AlH3O3 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 0 |
| Exact Mass | 77.9897574 g/mol |
| Monoisotopic Mass | 77.9897574 g/mol |
| Topological Polar Surface Area | 3 Ų |
| Heavy Atom Count | 4 |
| Formal Charge | 0 |
| Complexity | 0 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 4 |
For relief of heartburn and acid indigestion.
Gastric-peptic disease occurs as a result of an imbalance between protective factors, such as mucus, bicarbonate, and prostaglandin secretion, and aggressive factors, such as hydrochloric acid, pepsin, and Helicobacter pylori (H. pylori). Antacids work by restoring acid-base balance, attenuating the pepsin activity and increasing bicarbonate and prostaglandin secretion.
Absorption
Approximately 17-30% of the aluminum chloride formed is absorbed.
Route of Elimination
Absorbed aluminum chloride is rapidly eliminated by the kidneys in patients with normal renal function.
Not metabolized.
Aluminum hydroxide is a basic inorganic salt that acts by neutralizing hydrochloric acid in gastric secretions. Aluminum hydroxide is slowly solubilized in the stomach and reacts with hydrochloric acid to form aluminum chloride and water. It also inhibits the action of pepsin by increasing the pH and via adsorption. Cytoprotective effects may occur through increases in bicarbonate ion (HCO3-) and prostaglandins.






