



API Suppliers

US DMFs Filed

CEP/COS Certifications

JDMFs Filed
0
Other Certificates
Other Suppliers

USA (Orange Book)
0

Europe

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
U.S. Medicaid
0
Annual Reports
0
0
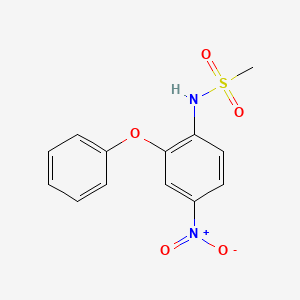

1. 4-nitro-2-phenoxymethanesulfonanilide
2. Antifloxil
3. Aulin
4. Eskaflam
5. Guaxan
6. Lizepat
7. Mesulid
8. Nexen
9. Nimesil
10. R 805
11. R-805
12. Redaflam
1. 51803-78-2
2. N-(4-nitro-2-phenoxyphenyl)methanesulfonamide
3. Mesulid
4. Aulin
5. Flogovital
6. Sulidene
7. Nimed
8. 4-nitro-2-phenoxymethanesulfonanilide
9. R-805
10. Methanesulfonamide, N-(4-nitro-2-phenoxyphenyl)-
11. R 805
12. Nisulid
13. 4'-nitro-2'-phenoxymethanesulfonanilide
14. 4-nitro-2-phenoxy-methanesulfonanilide
15. Nsc-758412
16. Chembl56367
17. Mls000069680
18. V4tkw1454m
19. Methanesulfonanilide, 4'-nitro-2'-phenoxy-
20. Chebi:44445
21. Ncgc00015725-02
22. R805
23. Smr000058484
24. Cas-51803-78-2
25. Dsstox_cid_17250
26. Dsstox_rid_79316
27. Dsstox_gsid_37250
28. Antifloxil
29. Nimesulida
30. Nimesulidum
31. Guaxan
32. Nimesulidum [inn-latin]
33. Nimesulida [inn-spanish]
34. Nim
35. Nimesulide [inn:ban]
36. Sr-01000000218
37. Einecs 257-431-4
38. 4'-nitro-2'-phenoxymethansulfonanilid
39. Brn 2421175
40. Unii-v4tkw1454m
41. Aldoron
42. Nimedex
43. Orthobid
44. Ccris 8225
45. Nise Gel
46. Nimesulide,(s)
47. Prestwick_618
48. Mfcd00079470
49. Spectrum_001577
50. Nimesulide [mi]
51. Nimesulide (jan/inn)
52. Nimesulide [inn]
53. Nimesulide [jan]
54. Opera_id_1247
55. Prestwick0_000194
56. Prestwick1_000194
57. Prestwick2_000194
58. Prestwick3_000194
59. Spectrum2_001541
60. Spectrum3_001576
61. Spectrum4_000178
62. Spectrum5_000964
63. Lopac-n-1016
64. N-(4-nitro-2-phenoxy-phenyl)methanesulfonamide
65. Nimesulide [mart.]
66. Nimesulide [who-dd]
67. Lopac0_000855
68. Schembl24882
69. Bspbio_000147
70. Bspbio_001103
71. Bspbio_003112
72. Kbiogr_000443
73. Kbiogr_000695
74. Kbioss_000443
75. Kbioss_002057
76. Mls001148268
77. Divk1c_000693
78. Spectrum1503231
79. Spbio_001382
80. Spbio_002068
81. N-(4-nitro-2-phenoxyphenyl)
82. Bpbio1_000163
83. Gtpl7401
84. Nim-03
85. Dtxsid3037250
86. Nimesulide [ep Impurity]
87. Bcbcmap01_000034
88. Hms502c15
89. Kbio1_000693
90. Kbio2_000443
91. Kbio2_002057
92. Kbio2_003011
93. Kbio2_004625
94. Kbio2_005579
95. Kbio2_007193
96. Kbio3_000825
97. Kbio3_000826
98. Kbio3_002612
99. Nimesulide [ep Monograph]
100. Nimesulide For Peak Identification
101. Ninds_000693
102. Bio2_000382
103. Bio2_000862
104. Hms1362g05
105. Hms1568h09
106. Hms1792g05
107. Hms1922k17
108. Hms1990g05
109. Hms2089b14
110. Hms2095h09
111. Hms2234k19
112. Hms3262l11
113. Hms3269g17
114. Hms3371j19
115. Hms3403g05
116. Hms3414p09
117. Hms3649a04
118. Hms3655d13
119. Hms3678p07
120. Hms3712h09
121. Hms3884c22
122. Pharmakon1600-01503231
123. Bcp10076
124. Hy-b0363
125. Zinc4617749
126. Tox21_110207
127. Tox21_301850
128. Tox21_500855
129. Bdbm50056999
130. Ccg-39319
131. Ei-287
132. Nsc758412
133. S2040
134. Stl018679
135. Akos015897356
136. Tox21_110207_1
137. Ac-4524
138. Db04743
139. Ks-1277
140. Lp00855
141. Nsc 758412
142. Sdccgsbi-0050831.p004
143. Idi1_000693
144. Idi1_002137
145. Ncgc00015725-01
146. Ncgc00015725-03
147. Ncgc00015725-04
148. Ncgc00015725-05
149. Ncgc00015725-06
150. Ncgc00015725-07
151. Ncgc00015725-08
152. Ncgc00015725-09
153. Ncgc00015725-10
154. Ncgc00015725-11
155. Ncgc00015725-12
156. Ncgc00015725-13
157. Ncgc00015725-15
158. Ncgc00015725-16
159. Ncgc00015725-29
160. Ncgc00021842-03
161. Ncgc00021842-04
162. Ncgc00021842-05
163. Ncgc00021842-06
164. Ncgc00021842-07
165. Ncgc00021842-08
166. Ncgc00255661-01
167. Ncgc00261540-01
168. Bn166246
169. Nimesulide 100 Microg/ml In Acetonitrile
170. Sbi-0050831.p003
171. Db-052029
172. Ab00052332
173. Eu-0100855
174. Ft-0630650
175. N0984
176. Sw196785-3
177. D01049
178. D70376
179. N 1016
180. Q20994
181. Ab00052332-16
182. Ab00052332_17
183. Ab00052332_18
184. N-[4-nitro-2-(phenoxy)phenyl]methanesulfonamide
185. 803n782
186. A828786
187. Sr-01000000218-2
188. Sr-01000000218-6
189. Sr-01000000218-7
190. W-105866
191. Brd-k76775527-001-06-2
192. Brd-k76775527-001-18-7
193. Sr-01000000218-11
194. Nimesulide, European Pharmacopoeia (ep) Reference Standard
195. Nimesulide, Pharmaceutical Secondary Standard; Certified Reference Material
196. Nimesulide For Peak Identification, European Pharmacopoeia (ep) Reference Standard
197. 1364966-82-4
| Molecular Weight | 308.31 g/mol |
|---|---|
| Molecular Formula | C13H12N2O5S |
| XLogP3 | 2.6 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 4 |
| Exact Mass | 308.04669266 g/mol |
| Monoisotopic Mass | 308.04669266 g/mol |
| Topological Polar Surface Area | 110 Ų |
| Heavy Atom Count | 21 |
| Formal Charge | 0 |
| Complexity | 450 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For the treatment of acute pain, the symptomatic treatment of osteoarthritis and primary dysmenorrhoea in adolescents and adults above 12 years old.
Food, gender and advanced age have negligible effects on nimesulide pharmacokinetics.
Anti-Inflammatory Agents, Non-Steroidal
Anti-inflammatory agents that are non-steroidal in nature. In addition to anti-inflammatory actions, they have analgesic, antipyretic, and platelet-inhibitory actions. They act by blocking the synthesis of prostaglandins by inhibiting cyclooxygenase, which converts arachidonic acid to cyclic endoperoxides, precursors of prostaglandins. Inhibition of prostaglandin synthesis accounts for their analgesic, antipyretic, and platelet-inhibitory actions; other mechanisms may contribute to their anti-inflammatory effects. (See all compounds classified as Anti-Inflammatory Agents, Non-Steroidal.)
Cyclooxygenase Inhibitors
Compounds or agents that combine with cyclooxygenase (PROSTAGLANDIN-ENDOPEROXIDE SYNTHASES) and thereby prevent its substrate-enzyme combination with arachidonic acid and the formation of eicosanoids, prostaglandins, and thromboxanes. (See all compounds classified as Cyclooxygenase Inhibitors.)
M - Musculo-skeletal system
M01 - Antiinflammatory and antirheumatic products
M01A - Antiinflammatory and antirheumatic products, non-steroids
M01AX - Other antiinflammatory and antirheumatic agents, non-steroids
M01AX17 - Nimesulide
M - Musculo-skeletal system
M02 - Topical products for joint and muscular pain
M02A - Topical products for joint and muscular pain
M02AA - Antiinflammatory preparations, non-steroids for topical use
M02AA26 - Nimesulide
Absorption
Rapidly absorbed following oral administration.
Route of Elimination
Renal (50%), fecal (29%)
Hepatic. Extensive biotransformation, mainly to 4-hydroxynimesulide (which also appears to be biologically active).
1.84.7 hours
The therapeutic effects of Nimesulide are the result of its complete mode of action which targets a number of key mediators of the inflammatory process such as: COX-2 mediated prostaglandins, free radicals, proteolytic enzymes and histamine.


