



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
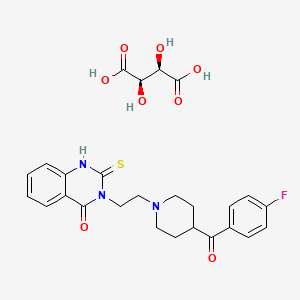

1. Altanserin
1. 79449-96-0
2. Altanserin Tartrate [usan]
3. 9p204che8j
4. 4(1h)-quinazolinone, 3-(2-(4-(4-fluorobenzoyl)-1-piperidinyl)ethyl)-2,3-dihydro-2-thioxo-, (r-(r*,r*))-2,3-dihydroxybutanedioate
5. Altanserin Tartrate (usan)
6. 3-(2-(4-(p-fluorobenzoyl)piperidino)ethyl)-2-thio-2,4(1h,3h)-quinazolinedione L-(+)-tartrate (1:1)
7. R-53,200
8. Unii-9p204che8j
9. R-53200
10. Einecs 279-159-5
11. Schembl124036
12. Dtxsid20229675
13. D02836
14. Q27272848
15. (2r,3r)-2,3-dihydroxybutanedioic Acid;3-[2-[4-(4-fluorobenzoyl)piperidin-1-yl]ethyl]-2-sulfanylidene-1h-quinazolin-4-one
16. 3-(2-(4-(4-fluorobenzoyl)piperidino)ethyl)-2,3-dihydro-2-thioxoquinazolin-4(1h)-one (r-(r*,r*))-tartrate
| Molecular Weight | 561.6 g/mol |
|---|---|
| Molecular Formula | C26H28FN3O8S |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 11 |
| Rotatable Bond Count | 8 |
| Exact Mass | 561.15811420 g/mol |
| Monoisotopic Mass | 561.15811420 g/mol |
| Topological Polar Surface Area | 200 Ų |
| Heavy Atom Count | 39 |
| Formal Charge | 0 |
| Complexity | 763 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Contrast Media
Substances used to allow enhanced visualization of tissues. (See all compounds classified as Contrast Media.)
Serotonin Antagonists
Drugs that bind to but do not activate serotonin receptors, thereby blocking the actions of serotonin or SEROTONIN RECEPTOR AGONISTS. (See all compounds classified as Serotonin Antagonists.)


