



API Suppliers

US DMFs Filed

CEP/COS Certifications

JDMFs Filed
0
Other Certificates
Other Suppliers

USA (Orange Book)

Europe
0

Canada

Australia
0

South Africa
Uploaded Dossiers
U.S. Medicaid
0
Annual Reports
0
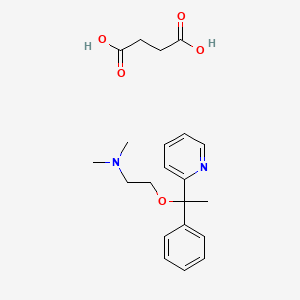

1. 562-10-7
2. Decapryn
3. Doxylamine (succinate)
4. Gittalun
5. Unisom
6. Doxylamine Hydrogen Succinate
7. Doxylamine Succinate Salt
8. Hoggar N
9. Doxy-sleep-aid
10. Evigoa D
11. A-h Injection
12. Decapryn Succinate
13. Doxylamine Succinate (1:1)
14. Alsadorm
15. Mereprine
16. Decapryn Succinate (1:1)
17. Alsodorm
18. Donormil
19. Donormyl
20. Dormidina
21. Meraprina
22. Nsc-74772
23. Doxylamine Succinate [usp]
24. V9bi9b5yi2
25. Butanedioic Acid;n,n-dimethyl-2-(1-phenyl-1-pyridin-2-ylethoxy)ethanamine
26. Mls000028417
27. 2-dimethylaminoethoxyphenylmethyl-2-picoline Succinate (1:1)
28. Chebi:82461
29. N,n-dimethyl-2-(1-phenyl-1-(pyridin-2-yl)ethoxy)ethanamine Succinate
30. Sedaplus
31. Smr000058436
32. Decapryn (tn)
33. Dsstox_cid_552
34. Doxylamine Succinate (usp)
35. Butanedioic Acid, Compd. With N,n-dimethyl-2-(1-phenyl-1-(2-pyridinyl)ethoxy)ethanamine (1:1)
36. Dsstox_rid_75656
37. Ethanamine, N,n-dimethyl-2-(1-phenyl-1-(2-pyridinyl)ethoxy)-, Butanedioate (1:1)
38. Dsstox_gsid_20552
39. Butanedioic Acid, Compd. Withn,n-dimethyl-2-[1-phenyl-1-(2-pyridinyl)ethoxy]ethanamine (1:1)other Ca Index Names:ethanamine, N,n-dimethyl-2-[1-phenyl-1-(2-pyridinyl)ethoxy]-,butanedioate (1:1)
40. N,n-dimethyl-2-(1-phenyl-1-pyridin-2-ylethoxy)ethanamine Succinate
41. Topcare Sleep Aid
42. Butanedioic Acid, Compd. With N,n-dimethyl-2-[1-phenyl-1-(2-pyridinyl)ethoxy]ethanamine (1:1)
43. Ccris 4811
44. Sr-01000003036
45. Einecs 209-228-7
46. Equaline Sleep Aid
47. Mfcd00056168
48. Nsc 74772
49. Doxylamine Succinate Liquid
50. Unii-v9bi9b5yi2
51. Safetussin
52. Ai3-23993
53. Ncgc00017028-02
54. Cas-562-10-7
55. Prestwick_887
56. Dimethylaminoethoxy-methyl-benzyl-pyridine Succinate
57. Decamium
58. 2-dimethylaminoethoxyphenylmethyl-2-picoline Succinate
59. Opera_id_566
60. Phenyl2-pyridylmethyl-beta-n,n-dimethylaminoethyl Ether Succinate
61. 2-(alpha-(2-dimethylaminoethoxy)-alpha-methylbenzyl)pyridine Succinate
62. 2-(alpha(2-(dimethylamino)ethoxy)-alpha-methylbenzyl)pyridine Succinate (1:1)
63. Alpha-(2-dimethylaminoethoxy)-alpha-methyl-alpha-phenyl-2-picoline Acid Succinate
64. Pyridine, Succinate (1:1)
65. Mls000758288
66. Mls001076141
67. Mls001424137
68. Mls002222274
69. Schembl160443
70. Spectrum1500267
71. Chembl1200392
72. Dtxsid7020552
73. Doxylamine For System Suitability
74. Hms502k03
75. Doxylamine Succinate [mi]
76. Hms1568e15
77. Hms1920k04
78. Hms2051f14
79. Hms2091b09
80. Hms2095e15
81. Hms2231g05
82. Hms3261e17
83. Hms3370k04
84. Hms3393f14
85. Hms3652h19
86. Hms3712e15
87. Hms3885d13
88. Pharmakon1600-01500267
89. Doxylamine Succinate [iarc]
90. Bcp24067
91. Hy-a0069
92. Nsc74772
93. Doxylamine Succinate [vandf]
94. Tox21_113515
95. Tox21_202078
96. Tox21_302861
97. Tox21_500348
98. Ccg-38929
99. Doxylamine Succinate [mart.]
100. Nsc756752
101. S4240
102. Doxylamine Succinate [usp-rs]
103. Doxylamine Succinate [who-dd]
104. Akos025310916
105. Butanedioic Acid,n,n-dimethyl-2-(1-phenyl-1-pyridin-2-ylethoxy)ethanamine
106. Tox21_113515_1
107. Ac-4487
108. Ccg-101033
109. Cs-3232
110. Lp00348
111. Nc00283
112. Nsc-756752
113. Pyridine, 2-(alpha-(2-(dimethylamino)ethoxy)-alpha-methylbenzyl)-, Succinate (1:1)
114. Doxylamine Succinate [green Book]
115. Ncgc00016140-02
116. Ncgc00016140-03
117. Ncgc00016140-04
118. Ncgc00021147-11
119. Ncgc00089789-03
120. Ncgc00093785-01
121. Ncgc00093785-02
122. Ncgc00093785-03
123. Ncgc00256517-01
124. Ncgc00259627-01
125. Ncgc00261033-01
126. As-13661
127. Doxylamine Succinate [orange Book]
128. Succinic Acid, Compd. With 2-(alpha-(2-(dimethylamino)ethoxy)-alpha-methylbenzyl)pyridine (1:1)
129. Db-052868
130. Doxylamine Succinate [usp Monograph]
131. D5583
132. Eu-0100348
133. Ft-0625595
134. Ft-0667789
135. Sw197008-4
136. Wln: T6nj Bx1&r&o2n1&1 &ov2vo
137. Bonjesta Component Doxylamine Succinate
138. Diclegis Component Doxylamine Succinate
139. Bendectin Component Doxylamine Succinate
140. C19414
141. D 3775
142. D-9200
143. D02327
144. D81886
145. Diclectin Component Doxylamine Succinate
146. Doxylamine Succinate Component Of Bonjesta
147. Doxylamine Succinate Component Of Diclegis
148. Doxylamine Hydrogen Succinate [ep Impurity]
149. Doxylamine Succinate Component Of Bendectin
150. Doxylamine Hydrogen Succinate [ep Monograph]
151. Sr-01000003036-2
152. Sr-01000003036-6
153. Sr-01000003036-9
154. (dimethylamino)ethoxy-methyl-benzyl-pyridine Succinate
155. F2173-1155
156. [[[(2-dimethylamino)ethoxy]phenyl]methyl]-2-picoline Succinate
157. 2-[a-[2-(dimethylamino)ethoxy]-a-methylbenzyl]pyridine Succinate
158. Doxylamine Hydrogen Succinate 1.0 Mg/ml In Methanol (as Free Base)
159. Doxylamine Succinate, United States Pharmacopeia (usp) Reference Standard
160. N,n-dimethyl-2-(1-phenyl-1-(pyridin-2-yl)ethoxy)ethanaminesuccinate
161. Phenyl2-pyridylmethyl-(.beta.-n,n-dimethylamino)ethyl Ether Succinate
162. 2-(.alpha.(2-(dimethylamino)ethoxy)-.alpha.-methylbenzyl)pyridine Succinate (1:1)
163. 2-[[.alpha.-(2-dimethylamino)ethoxy]-.alpha.-methylbenzyl]pyridine Succinate
164. Butanedioic Acid,n-dimethyl-2-[1-phenyl-1-(2-pyridinyl)ethoxy]ethanamine (1:1)
165. Doxylamine For System Suitability, European Pharmacopoeia (ep) Reference Standard
166. Doxylamine Hydrogen Succinate, European Pharmacopoeia (ep) Reference Standard
167. Doxylamine Succinate, Pharmaceutical Secondary Standard; Certified Reference Material
168. Doxylamine Succinate Solution, 1.0 Mg/ml In Methanol (as Free Base), Ampule Of 1 Ml, Certified Reference Material
169. N,n-dimethyl-2-((1rs)-1-phenyl-1-(pyridin-2-yl)ethoxy(ethanamine Hydrogen Butanedioate
170. Succinic Acid, Compd. With 2-[.alpha.-[2-(dimethylamino)ethoxy]-.alpha.-methylbenzyl]pyridine (1:1)
| Molecular Weight | 388.5 g/mol |
|---|---|
| Molecular Formula | C21H28N2O5 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 9 |
| Exact Mass | 388.19982200 g/mol |
| Monoisotopic Mass | 388.19982200 g/mol |
| Topological Polar Surface Area | 100 Ų |
| Heavy Atom Count | 28 |
| Formal Charge | 0 |
| Complexity | 369 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 4 | |
|---|---|
| Drug Name | Doxylamine succinate |
| Drug Label | TAMPER EVIDENT: Do not use if printed foil inner seal under bottle cap is broken or missing. Failure to follow these warnings could result in serious consequences.www.sambrosadreams.comProcessed in the United States with Foreign IngredientsDist. by S... |
| Active Ingredient | Doxylamine succinate |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 25mg |
| Market Status | Over the Counter |
| Company | Perrigo; Lnk |
| 2 of 4 | |
|---|---|
| Drug Name | Unisom |
| PubMed Health | Doxylamine (By mouth) |
| Drug Classes | Respiratory Agent |
| Active Ingredient | Doxylamine succinate |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 25mg |
| Market Status | Over the Counter |
| Company | Chattem |
| 3 of 4 | |
|---|---|
| Drug Name | Doxylamine succinate |
| Drug Label | TAMPER EVIDENT: Do not use if printed foil inner seal under bottle cap is broken or missing. Failure to follow these warnings could result in serious consequences.www.sambrosadreams.comProcessed in the United States with Foreign IngredientsDist. by S... |
| Active Ingredient | Doxylamine succinate |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 25mg |
| Market Status | Over the Counter |
| Company | Perrigo; Lnk |
| 4 of 4 | |
|---|---|
| Drug Name | Unisom |
| PubMed Health | Doxylamine (By mouth) |
| Drug Classes | Respiratory Agent |
| Active Ingredient | Doxylamine succinate |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 25mg |
| Market Status | Over the Counter |
| Company | Chattem |
Histamine H1 Antagonists
Drugs that selectively bind to but do not activate histamine H1 receptors, thereby blocking the actions of endogenous histamine. Included here are the classical antihistaminics that antagonize or prevent the action of histamine mainly in immediate hypersensitivity. They act in the bronchi, capillaries, and some other smooth muscles, and are used to prevent or allay motion sickness, seasonal rhinitis, and allergic dermatitis and to induce somnolence. The effects of blocking central nervous system H1 receptors are not as well understood. (See all compounds classified as Histamine H1 Antagonists.)






