



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
Other Suppliers
0

USA (Orange Book)

Europe

Canada

Australia
0

South Africa
Uploaded Dossiers
U.S. Medicaid
Annual Reports
0
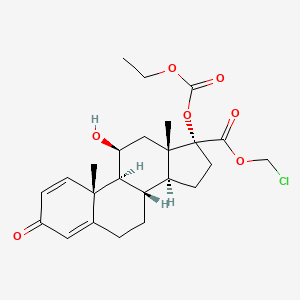

1. 17-ethoxycarbonyloxy-11-hydroxy-3-oxoandrosta-1,4-diene-17-carboxylate, Chloromethyl
2. Alrex
3. Cehoac
4. Chloromethyl 17 Ethoxycarbonyloxy 11 Hydroxy 3 Oxoandrosta 1,4 Diene 17 Carboxylate
5. Chloromethyl 17-ethoxycarbonyloxy-11-hydroxy-3-oxoandrosta-1,4-diene-17-carboxylate
6. Etabonate, Loteprednol
7. Lotemax
8. Loteprednol
1. 82034-46-6
2. Lotemax
3. Alrex
4. Hgp-1
5. Inveltys
6. Cddd-5604
7. P-5604
8. Cddd 5604
9. Chloromethyl (8s,9s,10r,11s,13s,14s,17r)-17-ethoxycarbonyloxy-11-hydroxy-10,13-dimethyl-3-oxo-7,8,9,11,12,14,15,16-octahydro-6h-cyclopenta[a]phenanthrene-17-carboxylate
10. Chebi:31784
11. Yeh1ez96k6
12. Kpi-121
13. Dsstox_cid_26468
14. Dsstox_rid_81641
15. Dsstox_gsid_46468
16. (11b,17a)-17-[(ethoxycarbonyl)oxy]-11-hydroxy-3-oxo-androsta-1,4-diene-17-carboxylic Acid Chloromethyl Ester
17. Chloromethyl 17alpha-[(ethoxycarbonyl)oxy]-11beta-hydroxy-3-oxoandrosta-1,4-diene-17beta-carboxylate
18. Loterox
19. Locort
20. Lotemax (tn)
21. Cas-82034-46-6
22. Unii-yeh1ez96k6
23. Hgp 1
24. Loteprednol Etabonate [usan]
25. Airex
26. Loteprednol Etabonate (jan/usan)
27. Loteprednoletabonate
28. Ncgc00164594-01
29. Idr-90102
30. Idr-90103
31. Eysuvis
32. Loteprednol Etabonate Opphthalmic Suspension
33. P 5604
34. Schembl23907
35. Mls001424221
36. Gtpl7085
37. Chembl1200865
38. Dtxsid2046468
39. Hms2051f16
40. Hms2232j09
41. Hms3715n06
42. Loteprednol Etabonate [mi]
43. Loteprednol Etabonate [usan:jan]
44. Loteprednol Etabonate [jan]
45. Bcp28645
46. Zinc3920673
47. Tox21_112219
48. Bdbm50248301
49. Loteprednol Etabonate [vandf]
50. Mfcd00870765
51. S1669
52. Loteprednol Etabonate [mart.]
53. Akos005145741
54. Loteprednol Etabonate [who-dd]
55. Tox21_112219_1
56. Ccg-101041
57. Cs-0900
58. Db14596
59. Gs-3599
60. Nc00291
61. Loteprednol Etabonate, >=98% (hplc)
62. Ncgc00164594-02
63. Chloromethyl 11beta,17-dihydroxy-3-oxoandrosta-1,4-diene-17beta-carboxylate, 17-(ethyl Carbonate)
64. Hy-17358
65. Smr000469178
66. Loteprednol Etabonate [orange Book]
67. Zylet Component Loteprednol Etabonate
68. L0327
69. D01689
70. Loteprednol Etabonate Component Of Zylet
71. Ab00698349-05
72. Ab00698349-07
73. Ab00698349_08
74. 034l466
75. Q3837481
76. Androsta-1,4-diene-17-carboxylic Acid, 17-((ethoxycarbonyl)oxy)-11-hydroxy-3-oxo-, Chloromethyl Ester, (11.beta.,17.alpha.)-
77. Androsta-1,4-diene-17-carboxylic Acid, 17-((ethoxycarbonyl)oxy)-11-hydroxy-3-oxo-, Chloromethyl Ester, (11beta,17alpha)-
78. Chloromethyl (1s,2r,10s,11s,14r,15s,17s)-14-[(ethoxycarbonyl)oxy]-17-hydroxy-2,15-dimethyl-5-oxotetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadeca-3,6-diene-14-carboxylate
79. Chloromethyl 11.beta.,17-dihydroxy-3-oxoandrosta-1,4-diene-17.beta.-carboxylate, 17-(ethyl Carbonate)
| Molecular Weight | 466.9 g/mol |
|---|---|
| Molecular Formula | C24H31ClO7 |
| XLogP3 | 3.9 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 7 |
| Exact Mass | 466.1758310 g/mol |
| Monoisotopic Mass | 466.1758310 g/mol |
| Topological Polar Surface Area | 99.1 Ų |
| Heavy Atom Count | 32 |
| Formal Charge | 0 |
| Complexity | 882 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 7 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Alrex |
| Drug Label | ALREX (loteprednol etabonate ophthalmic suspension) contains a sterile, topical anti-inflammatory corticosteroid for ophthalmic use.Loteprednol etabonate is a white to off-white powder.Loteprednol etabonate is represented by the following structura... |
| Active Ingredient | Loteprednol etabonate |
| Dosage Form | Suspension/drops |
| Route | Ophthalmic |
| Strength | 0.2% |
| Market Status | Prescription |
| Company | Bausch And Lomb |
| 2 of 4 | |
|---|---|
| Drug Name | Lotemax |
| PubMed Health | Loteprednol (Into the eye) |
| Drug Classes | Ophthalmologic Agent |
| Drug Label | LOTEMAX (loteprednol etabonate ophthalmic suspension) contains a sterile, topical anti-inflammatory corticosteroid for ophthalmic use. Loteprednol etabonate is a white to off-white powder.Loteprednol etabonate is represented by the following struct... |
| Active Ingredient | Loteprednol etabonate |
| Dosage Form | Ointment; Gel; Suspension/drops |
| Route | Ophthalmic |
| Strength | 0.5% |
| Market Status | Prescription |
| Company | Bausch And Lomb |
| 3 of 4 | |
|---|---|
| Drug Name | Alrex |
| Drug Label | ALREX (loteprednol etabonate ophthalmic suspension) contains a sterile, topical anti-inflammatory corticosteroid for ophthalmic use.Loteprednol etabonate is a white to off-white powder.Loteprednol etabonate is represented by the following structura... |
| Active Ingredient | Loteprednol etabonate |
| Dosage Form | Suspension/drops |
| Route | Ophthalmic |
| Strength | 0.2% |
| Market Status | Prescription |
| Company | Bausch And Lomb |
| 4 of 4 | |
|---|---|
| Drug Name | Lotemax |
| PubMed Health | Loteprednol (Into the eye) |
| Drug Classes | Ophthalmologic Agent |
| Drug Label | LOTEMAX (loteprednol etabonate ophthalmic suspension) contains a sterile, topical anti-inflammatory corticosteroid for ophthalmic use. Loteprednol etabonate is a white to off-white powder.Loteprednol etabonate is represented by the following struct... |
| Active Ingredient | Loteprednol etabonate |
| Dosage Form | Ointment; Gel; Suspension/drops |
| Route | Ophthalmic |
| Strength | 0.5% |
| Market Status | Prescription |
| Company | Bausch And Lomb |
A number of prescription loteprednol etabonate ophthalmic products are specifically indicated for the treatment of post-operative inflammation and pain following ocular surgery.
FDA Label
Loteprednol etabonate (LE) belongs to a unique class of corticosteroids with potent anti-inflammatory effects designed to be active at the site of action. Animal studies have shown that LE has a binding affinity to steroid receptors that is 4.3 times greater than dexamethasone. This particular class of steroids consists of bioactive molecules whose in-vivo transformation to non-toxic substances can be predicted from their chemistry and knowledge of enzymatic pathways in the body. Cortienic acid is an inactive metabolite of hydrocortisone and analogs of cortienic acid are also devoid of corticosteroid activity. Specifically, LE is an ester derivative of one of these analogs, cortienic acid etabonate. In particular, LE possesses a metabolically labile 17 beta-chloromethyl ester function which was designed in order to be hydrolyzed to an inactive carboxylic acid moiety. This inactive metabolite is more hydrophilic and is thus readily eliminated from the body. LE also exhibits good ocular permeation properties and good skin permeation properties.
Anti-Allergic Agents
Agents that are used to treat allergic reactions. Most of these drugs act by preventing the release of inflammatory mediators or inhibiting the actions of released mediators on their target cells. (From AMA Drug Evaluations Annual, 1994, p475) (See all compounds classified as Anti-Allergic Agents.)
Absorption
Loteprednol etabonate (LE) demonstrates good ocular permeation properties as it is lipid soluble, allowing the agent to penetrate into cells with relative ease. Results from the ocular administration of loteprednol in normal, healthy volunteers have shown that there are low or undetectable concentrations of either unchanged material or its metabolite. Following twice-daily unilateral topical ocular dosing of LE for 14 days in healthy subjects, the plasma concentrations of loteprednol etabonate were below the limit of quantitation (1 ng/mL) at all time points. These finds suggest that limited, if any, systemic absorption of LE occurs.
Route of Elimination
Following systemic administration to rats, loteprednol etabonate is eliminated primarily via the biliary/faecal route, with most of the dose eliminated in the form of the metabolite, PJ-90.
Volume of Distribution
The only data available regarding the volume of distribution of loteprednol etabonate (LE) is the volume of distribution the agent demonstrated when administered to dogs - a value of 3.7 L/kg. It has been shown, however, that the topical ocular administration of LE distributes preferentially into the cellular components of blood.
Clearance
Loteprednol etabonate was slowly hydrolyzed in liver at clearance rates of 0.21 +/- 0.04 and 2.41 +/- 0.13 ml/h/kg in the liver and plasma, respectively.
Loteprednol etabonate (LE) is readily and extensively metabolized to two inactive metabolites, PJ-90 (1-cortienic acid) and PJ-91 (1-cortienic acid etabonate). Metabolism occurs locally in ocular tissues, and to the extent that loteprednol etabonate reaches the systemic circulation, likely the liver and other tissues into which it distributes. In particular, studies have demonstrated that LE (chloromethyl 17alpha-ethoxycarbonyloxy-11beta-hydroxy-3-oxoandrosta-1,4-diene) is rapidly hydrolyzed at the location of its 17beta-chloromethyl ester function by paraoxonase 1 in human plasma at the site of administration at the level of the affected eye tissue to the 17beta-carboxylate PJ-91 metabolite and PJ-90 metabolite. Both metabolites are considered inactive.
The terminal half-life of loteprednol etabonate as determined when administered intravenously at a dose of 5 mg/kg in the dog animal model is 2.8 hours.
Corticosteroids like loteprednol etabonate inhibit the inflammatory response to a variety of inciting agents and likely delay or slow healing. They inhibit the edema, fibrin deposition, capillary dilation, leukocyte migration, capillary proliferation, fibroblast proliferation, deposition of collagen, and scar formation that are commonly associated with inflammation. While glucocorticoids are known to bind to and activate the glucocorticoid receptor, the molecular mechanisms involved in glucocorticoid/glucocorticoid receptor-dependent modulation of inflammation are not clearly established. Moreover, corticosteroids are thought to inhibit prostaglandin production through several independent mechanisms. In particular, corticosteroids are thought to act by the induction of phospholipase A2 inhibitory proteins, collectively called lipocortins. It is postulated that these proteins control the biosynthesis of potent mediators of inflammation such as prostaglandins and leukotrienes by inhibiting the release of their common precursor arachidonic acid. Arachidonic acid is released from membrane phospholipids by phospholipase A2. The use of LE subsequently treats post-operative inflammation and pain following ocular surgery by managing the prostaglandin release, recruitment and travel of neutrophils and macrophages, and production of other inflammatory mediators that are intrinsically associated with the physical trauma of surgery.






