
Synopsis
Synopsis
0
EU WC
0
VMF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
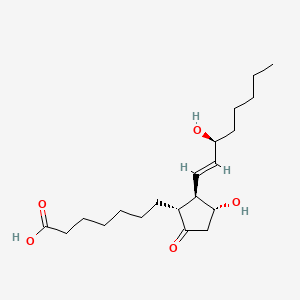

1. Caverject
2. Edex
3. Lipo Pge1
4. Lipo-pge1
5. Minprog
6. Muse
7. Pge1
8. Pge1alpha
9. Prostaglandin E1
10. Prostaglandin E1alpha
11. Prostavasin
12. Prostin Vr
13. Prostine Vr
14. Sugiran
15. Vasaprostan
16. Viridal
1. Prostaglandin E1
2. 745-65-3
3. Pge1
4. Edex
5. Caverject
6. Muse
7. Prostin Vr
8. Befar
9. Alprostadilum
10. Topiglan
11. Femprox
12. Alprox-td
13. L-prostaglandin E1
14. Liprostin
15. Prostandin
16. Vitaros
17. Prostin Vr Pediatric
18. Prink
19. Pge-1
20. Prostavasin
21. (11alpha,13e,15s)-11,15-dihydroxy-9-oxoprost-13-en-1-oic Acid
22. 7-((1r,2r,3r)-3-hydroxy-2-((s,e)-3-hydroxyoct-1-en-1-yl)-5-oxocyclopentyl)heptanoic Acid
23. Alprostadil(caverject)
24. (-)-prostaglandin E1
25. (13e)-(15s)-11alpha,15-dihydroxy-9-oxoprost-13-enoate
26. 11alpha,15alpha-dihydroxy-9-oxo-13-trans-prostenoic Acid
27. Chembl495
28. U-10136
29. 7-[(1r,2r,3r)-3-hydroxy-2-[(e,3s)-3-hydroxyoct-1-enyl]-5-oxocyclopentyl]heptanoic Acid
30. Vasaprostan
31. Chebi:15544
32. 9-oxo-11r,15s-dihydroxy-13e-prostaenoic Acid
33. Ono-1608
34. U-10,136
35. F5td010360
36. Nsc-165559
37. Minprog
38. Sugiran
39. Viridal
40. (13e,15s)-11alpha,15-dihydroxy-9-oxoprost-13-en-1-oic Acid
41. (1r,2r,3r)-3-hydroxy-2-((e)-(3s)-3-hydroxy-1-octenyl)-5-oxocyclopentaneheptanoic Acid
42. Lipoprost
43. Promostan
44. Prostivas
45. Alista
46. Femlife
47. Prostaglandine1
48. Rayva
49. Caverject Impulse
50. Pge1alpha
51. 7-((1r,2r,3r)-3-hydroxy-2-((s,e)-3-hydroxyoct-1-enyl)-5-oxocyclopentyl)heptanoic Acid
52. 7-[(1r,3r)-3-hydroxy-2-[(1e,3s)-3-hydroxyoct-1-en-1-yl]-5-oxocyclopentyl]heptanoic Acid
53. Smr000112594
54. Befar (tn)
55. Prink (tn)
56. Edex (tn)
57. Muse (tn)
58. (-)-protaglandin E1
59. U 10136
60. L-pge1
61. Sr-01000597593
62. Mr-256
63. Alprostadilum [inn-latin]
64. Prostin Vr Pediatric (tn)
65. Alprostadil Prostoglandin E1
66. Bml1-f06
67. Unii-f5td010360
68. Alprostadil(usan)
69. Hei-507
70. Ncgc00016535-01
71. Cas-745-65-3
72. Ono 1608
73. Einecs 212-017-2
74. (13e)-(15s)-11-alpha,15-dihydroxy-9-oxoprost-13-enoate
75. Mfcd00077860
76. Nsc 165559
77. Alprostadil [usan:usp:inn:ban:jan]
78. 11,15-dihydroxy-9-oxoprost-13-en-1-oic Acid
79. Pge1;prostaglandin E1
80. Ai3-62116
81. Prestwick2_001018
82. Prestwick3_001018
83. Alprostadil [inn]
84. Alprostadil [jan]
85. Dsstox_cid_2578
86. Alprostadil [usan]
87. Alprostadil [vandf]
88. Pge1 (prostaglandin E1)
89. Alprostadil [mart.]
90. Dsstox_rid_76640
91. Dsstox_gsid_22578
92. Schembl33317
93. Alprostadil [usp-rs]
94. Alprostadil [who-dd]
95. Bspbio_001175
96. Bspbio_001488
97. Mls000758964
98. Mls001424250
99. Bidd:gt0747
100. Prostaglandin E1 [mi]
101. Bpbio1_001293
102. Gtpl1882
103. Alprostadil (jp17/usp/inn)
104. Dtxsid9022578
105. Alprostadil [orange Book]
106. Pge1, Prostaglandin E1, Powder
107. Alprostadil [usp Impurity]
108. Hms1361k10
109. Hms1571k17
110. Hms1791k10
111. Hms1989k10
112. Hms2052l11
113. Hms2090l08
114. Hms2098k17
115. Hms3268i09
116. Hms3402k10
117. Hms3414n09
118. Hms3648o17
119. Hms3678n07
120. Hms3715k17
121. Alprostadil [usp Monograph]
122. Amy30076
123. Bcp01740
124. Ex-a1411
125. Hy-b0131
126. Zinc3813088
127. Tox21_110482
128. 3-hydroxy-2-(3-hydroxy-1-octenyl)-5-oxo-cyclopentaneheptanoic Acid
129. 3-hydroxy-2-(3-hydroxy-1-octenyl)-5-oxocyclopentaneheptanoic Acid
130. Bdbm50101853
131. L-3-hydroxy-2-(3-hydroxy-1-octenyl)-5-oxocyclopentaneheptanoic Acid
132. Lmfa03010134
133. S1508
134. Akos015961103
135. Prost-13-en-1-oic Acid, 11,15-dihydroxy-9-oxo-, (11.alpha.,13e,15s)-
136. Prost-13-en-1-oic Acid, 11,15-dihydroxy-9-oxo-, (11alpha,13e,15s)-
137. Ac-6095
138. Bcp9000277
139. Ccg-101188
140. Cs-1905
141. Db00770
142. Nc00438
143. Idi1_033958
144. Prostaglandin E1, >=99.0% (tlc)
145. Smp2_000271
146. Ncgc00025234-02
147. Ncgc00025234-03
148. Ncgc00025234-04
149. Ncgc00025234-05
150. As-16360
151. Prostaglandin E1, Pge1, 745-65-3
152. Alprostadil 100 Microg/ml In Acetonitrile
153. 11,15-dihydroxy-9-oxoprost-13-en-1-oate
154. Ab00514004
155. B2154
156. P1917
157. Alprostadil, Meets Usp Testing Specifications
158. C04741
159. C76381
160. D00180
161. Prostaglandin E1, >=98% (hplc), Synthetic
162. Ab00514004-06
163. Ab00514004-08
164. Ab00514004_09
165. 11,15-dihydroxy-9-oxoprost-13-en-1-oic Acidl
166. 745p653
167. A838163
168. Q579348
169. Sr-01000946253
170. Sr-01000597593-1
171. Sr-01000597593-5
172. Sr-01000597593-6
173. Sr-01000946253-1
174. W-104416
175. Brd-k52459643-001-06-0
176. Brd-k52459643-001-10-2
177. Brd-k52459643-001-17-7
178. (13e)-(15s)-11,15-dihydroxy-9-oxoprost-13-enoate
179. (13e)-(15s)-11,15-dihydroxy-9-oxoprost-13-enoic Acid
180. Alprostadil, European Pharmacopoeia (ep) Reference Standard
181. (13e)-(15s)-11alpha,15-dihydroxy-9-oxoprost-13-enoic Acid
182. (11?,13e,15s)-11,15-dihydroxy-9-oxo-prost-13-en-1-oic Acid
183. (13e)-(15s)-11-alpha,15-dihydroxy-9-oxoprost-13-enoic Acid
184. 11,15-dihydroxy-9-oxoprost-13-en-1-oic Acid (acd/name 4.0)
185. 3-hydroxy-2-(3-hydroxy-1-octenyl)-5-oxo-cyclopentaneheptanoate
186. Alprostadil, United States Pharmacopeia (usp) Reference Standard
187. (+)-3-hydroxy-2-(3-hydroxy-1-octenyl)-5-oxo-cyclopentaneheptanoate
188. (+)-3-hydroxy-2-(3-hydroxy-1-octenyl)-5-oxo-cyclopentaneheptanoic Acid
189. (-)-3-hydroxy-2-(3-hydroxy-1-octenyl)-5-oxo-cyclopentaneheptanoate
190. (-)-3-hydroxy-2-(3-hydroxy-1-octenyl)-5-oxo-cyclopentaneheptanoic Acid
191. (11alpha,12alpha,13e,15s)-11,15-dihydroxy-9-oxoprost-13-en-1-oic Acid
192. Cyclopentaneheptanoic Acid, 3-hydroxy-2-(3-hydroxy-1-octenyl)-5-oxo-, L-
193. Prost-13-en-1-oic Acid, 11,15-dihydroxy-9-oxo-, (11a,13e,15s)-
194. Prostaglandin E1, Synthetic, Powder, Bioreagent, Suitable For Cell Culture
195. Cyclopentaneheptanoic Acid, 3-hydroxy-2-(3-hydroxy-1-octenyl)-5-oxo-, (-)-
196. Prostaglandin E1, Powder, Gamma-irradiated, Bioxtra, Suitable For Cell Culture
197. Xpg
| Molecular Weight | 354.5 g/mol |
|---|---|
| Molecular Formula | C20H34O5 |
| XLogP3 | 3.2 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 13 |
| Exact Mass | 354.24062418 g/mol |
| Monoisotopic Mass | 354.24062418 g/mol |
| Topological Polar Surface Area | 94.8 Ų |
| Heavy Atom Count | 25 |
| Formal Charge | 0 |
| Complexity | 432 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 12 | |
|---|---|
| Drug Name | Caverject |
| PubMed Health | Alprostadil |
| Drug Classes | Endocrine-Metabolic Agent, Erectile Dysfunction Agent, Prostaglandin |
| Drug Label | CAVERJECT contains alprostadil as the naturally occurring form of prostaglandin E1 (PGE1) and is designated chemically as (11,13E,15S)-11,15-dihydroxy-9-oxoprost-13-en-1-oic acid. The molecular weight is 354.49.Alprostadil is a white to off-white c... |
| Active Ingredient | Alprostadil |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 0.005mg/vial; 0.02mg/vial; 0.04mg/vial; 0.01mg/vial |
| Market Status | Prescription |
| Company | Pharmacia And Upjohn |
| 2 of 12 | |
|---|---|
| Drug Name | Caverject impulse |
| Drug Label | CAVERJECT contains alprostadil as the naturally occurring form of prostaglandin E1 (PGE1) and is designated chemically as (11,13E,15S)-11,15-dihydroxy-9-oxoprost-13-en-1-oic acid. The molecular weight is 354.49.Alprostadil is a white to off-white c... |
| Active Ingredient | Alprostadil |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 0.02mg/vial; 0.01mg/vial |
| Market Status | Prescription |
| Company | Pharmacia And Upjohn |
| 3 of 12 | |
|---|---|
| Drug Name | Edex |
| PubMed Health | Alprostadil |
| Drug Classes | Endocrine-Metabolic Agent, Erectile Dysfunction Agent, Prostaglandin |
| Drug Label | edex (alprostadil for injection) is a sterile, pyrogen-free powder containing alprostadil in an alfadex (-cyclodextrin) inclusion complex. Alprostadil is an endogenous substance known as prostaglandin E1 (PGE1). edex is supplied in single-dose,... |
| Active Ingredient | Alprostadil |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 0.02mg/vial; 0.04mg/vial; 0.01mg/vial |
| Market Status | Prescription |
| Company | Auxilium Pharms |
| 4 of 12 | |
|---|---|
| Drug Name | Muse |
| PubMed Health | Alprostadil |
| Drug Classes | Endocrine-Metabolic Agent, Erectile Dysfunction Agent, Prostaglandin |
| Drug Label | MUSE (alprostadil) is a single-use, medicated transurethral system for the delivery of alprostadil to the male urethra. Alprostadil is suspended in polyethylene glycol 1450 (as excipient) and is formed into a medicated pellet (micro-suppository mea... |
| Active Ingredient | Alprostadil |
| Dosage Form | Suppository |
| Route | Urethral |
| Strength | 0.5mg; 1mg; 0.25mg; 0.125mg |
| Market Status | Prescription |
| Company | Meda Pharms |
| 5 of 12 | |
|---|---|
| Drug Name | Prostin vr pediatric |
| PubMed Health | Alprostadil |
| Drug Classes | Endocrine-Metabolic Agent, Erectile Dysfunction Agent, Prostaglandin |
| Drug Label | PROSTIN VR PEDIATRIC Sterile Solution for intravascular infusion contains 500 micrograms alprostadil, more commonly known as prostaglandin E1, in 1.0 mL dehydrated alcohol.The chemical name for alprostadil is (11,13E,15S)-11,15 dihydroxy-9-oxo-pros... |
| Active Ingredient | Alprostadil |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 0.5mg/ml |
| Market Status | Prescription |
| Company | Pharmacia And Upjohn |
| 6 of 12 | |
|---|---|
| Drug Name | Alprostadil |
| PubMed Health | Alprostadil |
| Drug Classes | Endocrine-Metabolic Agent, Erectile Dysfunction Agent, Prostaglandin |
| Drug Label | Alprostadil injection, USP for intravascular infusion contains 500 micrograms alprostadil, more commonly known as prostaglandin E1, in 1 mL dehydrated alcohol. The chemical name for alprostadil is (1R,2R,3R)3-Hydroxy-2-[(E)-(3S)-3-hydroxy-1-octenyl]-... |
| Active Ingredient | Alprostadil |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 0.5mg/ml |
| Market Status | Prescription |
| Company | Teva Pharms Usa; Eurohlth Intl |
| 7 of 12 | |
|---|---|
| Drug Name | Caverject |
| PubMed Health | Alprostadil |
| Drug Classes | Endocrine-Metabolic Agent, Erectile Dysfunction Agent, Prostaglandin |
| Drug Label | CAVERJECT contains alprostadil as the naturally occurring form of prostaglandin E1 (PGE1) and is designated chemically as (11,13E,15S)-11,15-dihydroxy-9-oxoprost-13-en-1-oic acid. The molecular weight is 354.49.Alprostadil is a white to off-white c... |
| Active Ingredient | Alprostadil |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 0.005mg/vial; 0.02mg/vial; 0.04mg/vial; 0.01mg/vial |
| Market Status | Prescription |
| Company | Pharmacia And Upjohn |
| 8 of 12 | |
|---|---|
| Drug Name | Caverject impulse |
| Drug Label | CAVERJECT contains alprostadil as the naturally occurring form of prostaglandin E1 (PGE1) and is designated chemically as (11,13E,15S)-11,15-dihydroxy-9-oxoprost-13-en-1-oic acid. The molecular weight is 354.49.Alprostadil is a white to off-white c... |
| Active Ingredient | Alprostadil |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 0.02mg/vial; 0.01mg/vial |
| Market Status | Prescription |
| Company | Pharmacia And Upjohn |
| 9 of 12 | |
|---|---|
| Drug Name | Edex |
| PubMed Health | Alprostadil |
| Drug Classes | Endocrine-Metabolic Agent, Erectile Dysfunction Agent, Prostaglandin |
| Drug Label | edex (alprostadil for injection) is a sterile, pyrogen-free powder containing alprostadil in an alfadex (-cyclodextrin) inclusion complex. Alprostadil is an endogenous substance known as prostaglandin E1 (PGE1). edex is supplied in single-dose,... |
| Active Ingredient | Alprostadil |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 0.02mg/vial; 0.04mg/vial; 0.01mg/vial |
| Market Status | Prescription |
| Company | Auxilium Pharms |
| 10 of 12 | |
|---|---|
| Drug Name | Muse |
| PubMed Health | Alprostadil |
| Drug Classes | Endocrine-Metabolic Agent, Erectile Dysfunction Agent, Prostaglandin |
| Drug Label | MUSE (alprostadil) is a single-use, medicated transurethral system for the delivery of alprostadil to the male urethra. Alprostadil is suspended in polyethylene glycol 1450 (as excipient) and is formed into a medicated pellet (micro-suppository mea... |
| Active Ingredient | Alprostadil |
| Dosage Form | Suppository |
| Route | Urethral |
| Strength | 0.5mg; 1mg; 0.25mg; 0.125mg |
| Market Status | Prescription |
| Company | Meda Pharms |
| 11 of 12 | |
|---|---|
| Drug Name | Prostin vr pediatric |
| PubMed Health | Alprostadil |
| Drug Classes | Endocrine-Metabolic Agent, Erectile Dysfunction Agent, Prostaglandin |
| Drug Label | PROSTIN VR PEDIATRIC Sterile Solution for intravascular infusion contains 500 micrograms alprostadil, more commonly known as prostaglandin E1, in 1.0 mL dehydrated alcohol.The chemical name for alprostadil is (11,13E,15S)-11,15 dihydroxy-9-oxo-pros... |
| Active Ingredient | Alprostadil |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 0.5mg/ml |
| Market Status | Prescription |
| Company | Pharmacia And Upjohn |
| 12 of 12 | |
|---|---|
| Drug Name | Alprostadil |
| PubMed Health | Alprostadil |
| Drug Classes | Endocrine-Metabolic Agent, Erectile Dysfunction Agent, Prostaglandin |
| Drug Label | Alprostadil injection, USP for intravascular infusion contains 500 micrograms alprostadil, more commonly known as prostaglandin E1, in 1 mL dehydrated alcohol. The chemical name for alprostadil is (1R,2R,3R)3-Hydroxy-2-[(E)-(3S)-3-hydroxy-1-octenyl]-... |
| Active Ingredient | Alprostadil |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 0.5mg/ml |
| Market Status | Prescription |
| Company | Teva Pharms Usa; Eurohlth Intl |
For palliative, not definitive, therapy to temporarily maintain the patency of the ductus arteriosus until corrective or palliative surgery can be performed in neonates who have congenital heart defects and who depend upon the patent ductus for survival. Also for the treatment of erectile dysfunction due to neurogenic, vasculogenic, psychogenic, or mixed etiology.
FDA Label
Alprostadil (prostaglandin E1) is produced endogenously to relax vascular smooth muscle and cause vasodilation. In adult males, the vasodilatory effects of alprostadil on the cavernosal arteries and the trabecular smooth muscle of the corpora cavernosa result in rapid arteriolar inflow and expansion of the lacunar spaces within the corpora. As the expanded corporal sinusoids are compressed against the tunica albuginea, venous outflow through the subtunical vessels is impeded and penile rigidity develops. This is referred to as the corporal veno-occlusive mechanism. In infants, the vasodilatory effects of alprostadil increase pulmonary or systemic blood flow.
Vasodilator Agents
Drugs used to cause dilation of the blood vessels. (See all compounds classified as Vasodilator Agents.)
Urological Agents
Drugs used in the treatment of urological conditions and diseases such as URINARY INCONTINENCE and URINARY TRACT INFECTIONS. (See all compounds classified as Urological Agents.)
Platelet Aggregation Inhibitors
Drugs or agents which antagonize or impair any mechanism leading to blood platelet aggregation, whether during the phases of activation and shape change or following the dense-granule release reaction and stimulation of the prostaglandin-thromboxane system. (See all compounds classified as Platelet Aggregation Inhibitors.)
C - Cardiovascular system
C01 - Cardiac therapy
C01E - Other cardiac preparations
C01EA - Prostaglandins
C01EA01 - Alprostadil
G - Genito urinary system and sex hormones
G04 - Urologicals
G04B - Urologicals
G04BE - Drugs used in erectile dysfunction
G04BE01 - Alprostadil
Absorption
The absolute bioavailability of alprostadil has not been determined.
Route of Elimination
Alprostadil must be infused continuously because it is very rapidly metabolized. As much as 80% of the circulating alprostadil may be metabolized in one pass through the lungs, primarily by - and -oxidation. The metabolites are excreted primarily by the kidney, and excretion is essentially complete within 24 hours after administration.
Alprostadil must be infused continuously because it is very rapidly metabolized. As much as 80% of the circulating alprostadil may be metabolized in one pass through the lungs, primarily by beta- and omega-oxidation.
5 to 10 minutes (after a single dose), in healthy adults and neonates.
Alprostadil causes vasodilation by means of a direct effect on vascular and ductus arteriosus (DA) smooth muscle, preventing or reversing the functional closure of the DA that occurs shortly after birth. This is because, as a form of prostaglandinE1 (PGE1) it has multiple effects on blood flow. This results in increased pulmonary or systemic blood flow in infants. In cyanotic congenital heart disease, alprostadil's actions result in an increased oxygen supply to the tissues. In infants with interrupted aortic arch or very severe aortic coarctation, alprostadil maintains distal aortic perfusion by permitting blood flow through the DA from the pulmonary artery to the aorta. In infants with aortic coarctation, alprostadil reduces aortic obstruction either by relaxing ductus tissue in the aortic wall or by increasing effective aortic diameter by dilating the DA. In infants with these aortic arch anomalies, systemic blood flow to the lower body is increased, improving tissue oxygen supply and renal perfusion. When administered by intracavernosal injection or as an intraurethral suppository, alprostadil acts locally to relax the trabecular smooth muscle of the corpora cavernosa and the cavernosal arteries. Swelling, elongation, and rigidity of the penis result when arterial blood rapidly flows into the corpus cavernosum to expand the lacunar spaces. The entrapped blood reduces the venous blood outflow as sinusoids compress against the tunica albuginea.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
38
PharmaCompass offers a list of Alprostadil API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Alprostadil manufacturer or Alprostadil supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Alprostadil manufacturer or Alprostadil supplier.
PharmaCompass also assists you with knowing the Alprostadil API Price utilized in the formulation of products. Alprostadil API Price is not always fixed or binding as the Alprostadil Price is obtained through a variety of data sources. The Alprostadil Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Alprostadil Prostoglandin E1 manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Alprostadil Prostoglandin E1, including repackagers and relabelers. The FDA regulates Alprostadil Prostoglandin E1 manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Alprostadil Prostoglandin E1 API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Alprostadil Prostoglandin E1 manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Alprostadil Prostoglandin E1 supplier is an individual or a company that provides Alprostadil Prostoglandin E1 active pharmaceutical ingredient (API) or Alprostadil Prostoglandin E1 finished formulations upon request. The Alprostadil Prostoglandin E1 suppliers may include Alprostadil Prostoglandin E1 API manufacturers, exporters, distributors and traders.
click here to find a list of Alprostadil Prostoglandin E1 suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Alprostadil Prostoglandin E1 DMF (Drug Master File) is a document detailing the whole manufacturing process of Alprostadil Prostoglandin E1 active pharmaceutical ingredient (API) in detail. Different forms of Alprostadil Prostoglandin E1 DMFs exist exist since differing nations have different regulations, such as Alprostadil Prostoglandin E1 USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Alprostadil Prostoglandin E1 DMF submitted to regulatory agencies in the US is known as a USDMF. Alprostadil Prostoglandin E1 USDMF includes data on Alprostadil Prostoglandin E1's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Alprostadil Prostoglandin E1 USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Alprostadil Prostoglandin E1 suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Alprostadil Prostoglandin E1 Drug Master File in Japan (Alprostadil Prostoglandin E1 JDMF) empowers Alprostadil Prostoglandin E1 API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Alprostadil Prostoglandin E1 JDMF during the approval evaluation for pharmaceutical products. At the time of Alprostadil Prostoglandin E1 JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Alprostadil Prostoglandin E1 suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Alprostadil Prostoglandin E1 Drug Master File in Korea (Alprostadil Prostoglandin E1 KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Alprostadil Prostoglandin E1. The MFDS reviews the Alprostadil Prostoglandin E1 KDMF as part of the drug registration process and uses the information provided in the Alprostadil Prostoglandin E1 KDMF to evaluate the safety and efficacy of the drug.
After submitting a Alprostadil Prostoglandin E1 KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Alprostadil Prostoglandin E1 API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Alprostadil Prostoglandin E1 suppliers with KDMF on PharmaCompass.
A Alprostadil Prostoglandin E1 CEP of the European Pharmacopoeia monograph is often referred to as a Alprostadil Prostoglandin E1 Certificate of Suitability (COS). The purpose of a Alprostadil Prostoglandin E1 CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Alprostadil Prostoglandin E1 EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Alprostadil Prostoglandin E1 to their clients by showing that a Alprostadil Prostoglandin E1 CEP has been issued for it. The manufacturer submits a Alprostadil Prostoglandin E1 CEP (COS) as part of the market authorization procedure, and it takes on the role of a Alprostadil Prostoglandin E1 CEP holder for the record. Additionally, the data presented in the Alprostadil Prostoglandin E1 CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Alprostadil Prostoglandin E1 DMF.
A Alprostadil Prostoglandin E1 CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Alprostadil Prostoglandin E1 CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Alprostadil Prostoglandin E1 suppliers with CEP (COS) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Alprostadil Prostoglandin E1 as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Alprostadil Prostoglandin E1 API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Alprostadil Prostoglandin E1 as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Alprostadil Prostoglandin E1 and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Alprostadil Prostoglandin E1 NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Alprostadil Prostoglandin E1 suppliers with NDC on PharmaCompass.
Alprostadil Prostoglandin E1 Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Alprostadil Prostoglandin E1 GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Alprostadil Prostoglandin E1 GMP manufacturer or Alprostadil Prostoglandin E1 GMP API supplier for your needs.
A Alprostadil Prostoglandin E1 CoA (Certificate of Analysis) is a formal document that attests to Alprostadil Prostoglandin E1's compliance with Alprostadil Prostoglandin E1 specifications and serves as a tool for batch-level quality control.
Alprostadil Prostoglandin E1 CoA mostly includes findings from lab analyses of a specific batch. For each Alprostadil Prostoglandin E1 CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Alprostadil Prostoglandin E1 may be tested according to a variety of international standards, such as European Pharmacopoeia (Alprostadil Prostoglandin E1 EP), Alprostadil Prostoglandin E1 JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Alprostadil Prostoglandin E1 USP).

