



API Suppliers

US DMFs Filed

CEP/COS Certifications

JDMFs Filed
Other Certificates
Other Suppliers
0

USA (Orange Book)
0

Europe

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
U.S. Medicaid
0
Annual Reports
0
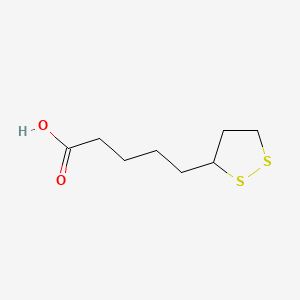

1. Acid, Alpha-lipoic
2. Alpha Lipogamma
3. Alpha Lipoic Acid
4. Alpha Lipon Stada
5. Alpha Liponaure Heumann
6. Alpha Liponsaure Sofotec
7. Alpha Liponsaure Von Ct
8. Alpha Lippon Al
9. Alpha Vibolex
10. Alpha-lipogamma
11. Alpha-lipoic Acid
12. Alpha-lipon Stada
13. Alpha-liponaure Heumann
14. Alpha-liponsaure Sofotec
15. Alpha-liponsaure Von Ct
16. Alpha-lippon Al
17. Alpha-vibolex
18. Alphaflam
19. Alphalipogamma
20. Alphalipon Stada
21. Alphaliponaure Heumann
22. Alphaliponsaure Sofotec
23. Alphaliponsaure Von Ct
24. Alphalippon Al
25. Alphavibolex
26. Azulipont
27. Biomo Lipon
28. Biomo-lipon
29. Biomolipon
30. Duralipon
31. Espa Lipon
32. Espa-lipon
33. Espalipon
34. Fenint
35. Injekt, Thiogamma
36. Juthiac
37. Lipoic Acid
38. Liponsaure Ratiopharm
39. Liponsaure-ratiopharm
40. Liponsaureratiopharm
41. Mtw Alphaliponsaure
42. Mtw-alphaliponsaure
43. Mtwalphaliponsaure
44. Neurium
45. Pleomix Alpha
46. Pleomix Alpha N
47. Pleomix-alpha
48. Pleomix-alpha N
49. Pleomixalpha
50. Pleomixalpha N
51. Thioctacid
52. Thioctacide T
53. Thiogamma Injekt
54. Thiogamma Oral
55. Tromlipon
56. Verla Lipon
57. Verla-lipon
58. Verlalipon
1. Dl-thioctic Acid
2. Alpha-lipoic Acid
3. 1077-28-7
4. 5-(1,2-dithiolan-3-yl)pentanoic Acid
5. Dl-alpha-lipoic Acid
6. 1,2-dithiolane-3-pentanoic Acid
7. Biletan
8. Alpha Lipoic Acid
9. 6,8-thioctic Acid
10. Thioctacid
11. Dl-6,8-thioctic Acid
12. 6-thioctic Acid
13. Lipothion
14. 62-46-4
15. 5-(dithiolan-3-yl)pentanoic Acid
16. Dl-lipoic Acid
17. Liposan
18. Thioctsan
19. Tioctacid
20. Rac-lipoate
21. 1,2-dithiolane-3-valeric Acid
22. 6,8-dithiooctanoic Acid
23. Alpha-liponsaeure
24. Dl-6-thioctic Acid
25. Thioctic Acid Dl-form
26. 5-(1,2-dithiolan-3-yl)valeric Acid
27. Alpha-liponic Acid
28. Thioktsaeure
29. Tioctidasi
30. (rs)-lipoic Acid
31. Espa-lipon
32. 5-(dithiolan-3-yl)valeric Acid
33. Acetate-replacing Factor
34. 6,8-thiotic Acid
35. Thioctansaeure
36. 6-thiotic Acid
37. .alpha.-lipoic Acid
38. Dl-1,2-dithiolane 3-valeric Acid
39. (+-)-lipoic Acid
40. Thioctate
41. Thioctic Acid [jan]
42. Thioctsaeure
43. Thiocacid
44. Thioctan
45. Liponic Acid
46. Thiooctic Acid
47. A-lipoic Acid
48. Dl-.alpha.-lipoic Acid
49. Acidum Thiocticum
50. (+/-)-1,2-dithiolane-3-pentanoic Acid
51. A-lipoicum Acidum
52. Dl-1,2-dithiolane-3-valeric Acid
53. Lipoate
54. Mfcd00005474
55. Nsc 90788
56. Lipoic Acid, Alpha
57. Lipoic Acid, Dl-
58. Nsc 628502
59. (+-)-1,2-dithiolane-3-pentanoic Acid
60. .alpha.-liponic Acid
61. Chebi:16494
62. Dl-1,2-dithiolan-3-valeriansaeure
63. (.+-.)-lipoic Acid
64. Nsc-90788
65. (rs)-.alpha.-lipoic Acid
66. 5-[3-(1,2-dithiolanyl)]pentanoic Acid
67. Chembl33864
68. Tioctic Acid
69. (.+-.)-.alpha.-lipoic Acid
70. 73y7p0k73y
71. 1,2-dithiolane-3-valeric Acid, (+-)-
72. Nsc90788
73. Nsc-628502
74. Thioctic Acid (jan)
75. Ncgc00016032-06
76. Dsstox_cid_5508
77. Protogen A
78. (+/-)-alpha-lipoic Acid
79. Dsstox_rid_77816
80. Dsstox_gsid_25508
81. (r)-(+)-alpha-lipoic Acid;r-(+)-thioctic Acid
82. Thioktsaeure [german]
83. Biomolipon
84. Duralipon
85. Alipure
86. Alphalipogamma
87. Thiotacid
88. Biomo Lipon
89. Espa Lipon
90. Alpha Lipogamma
91. Alpha-lipogamma
92. Pyruvate Oxidation Factor
93. Dl-thiocticacid
94. Pleomix Alpha
95. Thioctacide T
96. Verla Lipon
97. Alphalipon Stada
98. Alpha Lippon Al
99. Alpha-liponsaeure [german]
100. Alpha Lipon Stada
101. Alpha-lipon Stada
102. 5-(1,2)dithiolan-3-yl-pentanoic Acid
103. 5-[1,2]dithiolan-3-yl-pentanoic Acid
104. Liponsaureratiopharm
105. Alpha-lipon 300
106. Smr000058198
107. Cas-1077-28-7
108. Liponsaure-ratiopharm
109. (+-)-thioctic Acid
110. 5-(3-(1,2-dithiolanyl))pentanoic Acid
111. Alpha Liponsaure Von Ct
112. Tioctidasi Acetate Replacing Factor
113. Sr-01000737460
114. Dl-6,8-dithiooctanoic Acid
115. Lip(s2)
116. (rs)-alpha-lipoic Acid
117. Einecs 200-534-6
118. Einecs 214-071-2
119. (+-)-alpha-lipoic Acid
120. Brn 0081853
121. Brn 0122410
122. Unii-73y7p0k73y
123. Alpha Lipoic
124. Alphalipoic-acid
125. Dl-1,2-dithiolan-3-valeriansaeure [german]
126. Thioctic Acid [inn:ban:jan]
127. Hsdb 7818
128. Alpha-lipoic-acid
129. D,l-lipoic Acid
130. Thiotomin (tn)
131. Dl-a-lipoic Acid
132. D,l-thioctic Acid
133. Lipoic Acid (la)
134. Alpha -lipoic Acid
135. Rac ?-lipoic Acid
136. (rs)-thioctic Acid
137. Lipoic-acid
138. ()-alpha-lipoic Acid
139. Spectrum_001618
140. 5-(1,2-dithiolan-3-yl)-pentanoate
141. Thioctic Acid, Dl-form
142. R-(+)-alpalipoic Acid
143. 1,2-dithiolane-3-pentanoic Acid, (+-)-
144. Spectrum2_001605
145. Spectrum3_001188
146. Spectrum4_000217
147. Spectrum5_001298
148. (s)-(-)-thiocticacid
149. (+/-)-a-lipoic Acid
150. Cid_864
151. (.+-.)-thioctic Acid
152. Lipoic Acid, Alpha [nf]
153. Bmse000542
154. Epitope Id:150922
155. (+/-)-?-lipoic Acid
156. (.+/-.)-lipoic Acid
157. Thioctic Acid [mi]
158. Schembl51065
159. Bspbio_002835
160. Dl-thioctic Acid (oxidized)
161. Kbiogr_000853
162. Kbioss_002098
163. Thioctic Acid [hsdb]
164. Thioctic Acid [inci]
165. 5-19-07-00237 (beilstein Handbook Reference)
166. Mls000069736
167. Mls001332379
168. Mls001332380
169. Mls002153365
170. Divk1c_000912
171. Spectrum1503941
172. Spbio_001609
173. Thioctic Acid [mart.]
174. Thioctic Acid [who-dd]
175. Dtxsid7025508
176. Bdbm10515
177. Hms502n14
178. Kbio1_000912
179. Kbio2_002098
180. Kbio2_004666
181. Kbio2_007234
182. Kbio3_002335
183. A-lipoicum Acidum [hpus]
184. Alpha Lipoic Acid [vandf]
185. Alpha-lipoic Acid [vandf]
186. Ninds_000912
187. Thioctic Acid (alpha-lipoic Acid)
188. Hms1922m22
189. Hms3649h08
190. Hms3885i16
191. Pharmakon1600-01503941
192. Thioctic Acid, (+/-)-
193. Act14091
194. Albb-030318
195. Alpha Lipoic Acid [usp-rs]
196. Bcp13221
197. Bcp14048
198. Bcp18944
199. Hy-n0492
200. Thioctic Acid Dl-form [mi]
201. Tox21_110285
202. Tox21_201808
203. Tox21_303092
204. Ac7875
205. Bbl013878
206. Ccg-39063
207. Dl-1,2-dithiolane-3-pentanoic Acid
208. Nsc628502
209. Nsc758651
210. S3996
211. Stk801969
212. Thioctic Acid [ep Monograph]
213. ()-1,2-dithiolane-3-pentanoic Acid
214. Akos000121582
215. Akos016339634
216. Tox21_110285_1
217. Ab09328
218. Am84329
219. Cs-4370
220. Ks-1322
221. Nsc-758651
222. Sb49517
223. Idi1_000912
224. Alpha Lipoic Acid [usp Impurity]
225. Dl-thioctic Acid (oxidized) 25g
226. Ncgc00016032-02
227. Ncgc00016032-03
228. Ncgc00016032-04
229. Ncgc00016032-05
230. Ncgc00016032-07
231. Ncgc00016032-08
232. Ncgc00016032-09
233. Ncgc00016032-11
234. Ncgc00016032-14
235. Ncgc00090872-01
236. Ncgc00090872-02
237. Ncgc00090872-03
238. Ncgc00090872-04
239. Ncgc00090872-05
240. Ncgc00256970-01
241. Ncgc00259357-01
242. (+/-)-alpha-lipoic Acid, >=98.0%
243. .alpha.-lipoic Acid, (+/-)-
244. Ac-22673
245. Bp-31070
246. Nci60_042014
247. R)-(+)-
248. A-lipoic Acid Ooethyaoethaea
249. Sy010902
250. (r)-(+)-(c) Paragraph Sign-lipoic Acid
251. Sbi-0051871.p002
252. 5-(1,2-dithiolan-3-yl)pentanoic Acid #
253. Db-050522
254. ( Inverted Exclamation Marka)-a-lipoic Acid
255. 1,2-dithiolane-3-valeric Acid, (.+-.)-
256. Ft-0622068
257. Ft-0625429
258. Ft-0670812
259. Ft-0670813
260. L0058
261. 1,2-dithiolane-3-pentanoic Acid, (.+-.)-
262. 1,2-dithiolane-3-valeric Acid, (.+/-.)-
263. 1,2-dithiolane-3-pentanoic Acid, (.+/-.)-
264. C00725
265. D00086
266. Ab00052393_09
267. (+/-)?-?1,2-?dithiolane-?3-?pentanoic Acid
268. A801751
269. Q312229
270. 1,2-dithiolane-3-pentanoic Acid, (+-)- (9ci)
271. J-002007
272. J-520421
273. Sr-01000737460-2
274. Sr-01000737460-6
275. 5-((3rs)-1,2-dithiolan-3-yl)pentanoic Acid
276. F2191-0208
277. .delta.-(3-(1,2-dithiacyclopentyl))pentanoic Acid
278. Thioctic Acid, European Pharmacopoeia (ep) Reference Standard
279. (+/-)-alpha-lipoic Acid, Bioreagent, Cell Culture Tested, >=99%
280. (+/-)-alpha-lipoic Acid, Synthetic, >=99% (titration), Powder
281. Alpha Lipoic Acid, United States Pharmacopeia (usp) Reference Standard
282. (r)-(+)-1,2-dithiolane-3-pentanoic Acid; R-(+)-thioctic Acid; R-(+)-alpha-lipoic Acid
283. Afae'a Centa' Nota Inverted Exclamation Markafasa'a
284. Afae'adaggeratrade Mark?-lipoic Acid
285. Thioctic Acid Containing Impurity B, European Pharmacopoeia (ep) Reference Standard
286. Thioctic Acid For System Suitability, European Pharmacopoeia (ep) Reference Standard
287. Thioctic Acid;1,2-dithiolane-3-pentanoic Acid;5-(1,2-dithiolan-3-yl)valeric Acid
1. 1200-22-2
2. (r)-lipoic Acid
3. Heparlipon
4. Lipoec
5. Tioctic Acid
| Molecular Weight | 206.3 g/mol |
|---|---|
| Molecular Formula | C8H14O2S2 |
| XLogP3 | 1.7 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 5 |
| Exact Mass | 206.04352203 g/mol |
| Monoisotopic Mass | 206.04352203 g/mol |
| Topological Polar Surface Area | 87.9 Ų |
| Heavy Atom Count | 12 |
| Formal Charge | 0 |
| Complexity | 150 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
/EXPERIMENTAL THERAPY/ The aim of this trial was to evaluate the effects of alpha-lipoic acid (ALA) on positive sensory symptoms and neuropathic deficits in diabetic patients with distal symmetric polyneuropathy (DSP). In this multicenter, randomized, double-blind, placebo-controlled trial, 181 diabetic patients in Russia and Israel received once-daily oral doses of 600 mg (n = 45) (ALA600), 1,200 mg (n = 47) (ALA1200), and 1,800 mg (ALA1800) of ALA (n = 46) or placebo (n = 43) for 5 weeks after a 1-week placebo run-in period. The primary outcome measure was the change from baseline of the Total Symptom Score (TSS), including stabbing pain, burning pain, paresthesia, and asleep numbness of the feet. Secondary end points included individual symptoms of TSS, Neuropathy Symptoms and Change (NSC) score, Neuropathy Impairment Score (NIS), and patients' global assessment of efficacy. Mean TSS did not differ significantly at baseline among the treatment groups and on average decreased by 4.9 points (51%) in ALA600, 4.5 (48%) in ALA1200, and 4.7 (52%) in ALA1800 compared with 2.9 points (32%) in the placebo group (all P < 0.05 vs. placebo). The corresponding response rates (> or = 50% reduction in TSS) were 62, 50, 56, and 26%, respectively. Significant improvements favoring all three ALA groups were also noted for stabbing and burning pain, the NSC score, and the patients' global assessment of efficacy. The NIS was numerically reduced. Safety analysis showed a dose-dependent increase in nausea, vomiting, and vertigo. CONCLUSIONS: Oral treatment with ALA for 5 weeks improved neuropathic symptoms and deficits in patients with DSP. An oral dose of 600 mg once daily appears to provide the optimum risk-to-benefit ratio.
PMID:17065669 Ziegler D E et al; Diabetes Care 29 (11): 2365-70 (2006)
/EXPERIMENTAL THERAPY/ Mitochondria produce reactive oxygen species that may contribute to vascular dysfunction. alpha-Lipoic acid and acetyl-L-carnitine reduce oxidative stress and improve mitochondrial function. In a double-blind crossover study, the authors examined the effects of combined alpha-lipoic acid/acetyl-L-carnitine treatment and placebo (8 weeks per treatment) on vasodilator function and blood pressure in 36 subjects with coronary artery disease. Active treatment increased brachial artery diameter by 2.3% (P=.008), consistent with reduced arterial tone. Active treatment tended to decrease systolic blood pressure for the whole group (P=.07) and had a significant effect in the subgroup with blood pressure above the median (151+/-20 to 142+/-18 mm Hg; P=.03) and in the subgroup with the metabolic syndrome (139+/-21 to 130+/-18 mm Hg; P=.03). Thus, mitochondrial dysfunction may contribute to the regulation of blood pressure and vascular tone....
PMID:17396066 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2734271 McMackin CJ et al; J Clin Hypertens (Greenwich) 9 (4): 249-55 (2007)
/EXPERIMENTAL THERAPY/ Lipoic acid is an antioxidant that suppresses and treats an animal model of multiple sclerosis, experimental autoimmune encephalomyelitis. The purpose of this study was to determine the pharmacokinetics (PK), tolerability and effects on matrix metalloproteinase-9 (MMP-9) and soluble intercellular adhesion molecule-1 (sICAMP-1) of oral lipoic acid in patients with multiple sclerosis. Thirty-seven MS subjects were randomly assigned to one of four groups: placebo, lipoic acid 600 mg twice a day, lipoic acid 1200 mg once a day and lipoic acid 1200 mg twice a day. Subjects took study capsules for 14 days. ... Subjects taking 1200 mg lipoic acid had substantially higher peak serum lipoic acid levels than those taking 600 mg and that peak levels varied considerably among subjects. We also found a significant negative correlation between peak serum lipoic acid levels and mean changes in serum MMP-9 levels (T = -0.263, P =0.04). There was a significant dose response relationship between lipoic acid and mean change in serum sICAM-1 levels (P =0.03). ... Oral lipoic acid is generally well tolerated and appears capable of reducing serum MMP-9 and sICAM-1 levels. Lipoic acid may prove useful in treating MS by inhibiting MMP-9 activity and interfering with T-cell migration into the CNS.
PMID:15794388 Yadav V et al; Mult Scler 11 (2): 159-65 (2005)
/EXPERIMENTAL THERAPY/ Mitochondrial dysfunction and oxidative damage are highly involved in the pathogenesis of Parkinson's disease. Some mitochondrial antioxidants/nutrients that can improve mitochondrial function and/or attenuate oxidative damage have been implicated in Parkinson's disease therapy. However, few studies have evaluated the preventative effects of a combination of mitochondrial antioxidants/nutrients against Parkinson's disease, and even fewer have sought to optimize the doses of the combined agents. The present study examined the preventative effects of two mitochondrial antioxidant/nutrients, R-alpha-lipoic acid (LA) and acetyl-L-carnitine (ALC), in a chronic rotenone-induced cellular model of Parkinson's disease. We demonstrated that 4-week pretreatment with LA and/or ALC effectively protected SK-N-MC human neuroblastoma cells against rotenone-induced mitochondrial dysfunction, oxidative damage, and accumulation of alpha-synuclein and ubiquitin. Most notably, we found that when combined, LA and ALC worked at 100 to 1000 fold lower concentrations than they did individually. We also found that pretreatment with combined LA and ALC increased mitochondrial biogenesis and decreased production of reactive oxygen species through the upregulation of the peroxisome proliferator-activated receptor-gamma coactivator 1alpha as a possible underlying mechanism. This study provides important evidence that combining mitochondrial antioxidant/nutrients at optimal doses might be an effective and safe prevention strategy for Parkinson's disease. /R-alpha-lipoic acid/
PMID:18624765 Zhang H et al; J Cell Mol Med. 2008 Jun 20. (Epub ahead of print)
For more Therapeutic Uses (Complete) data for alpha-Lipoic acid (11 total), please visit the HSDB record page.
Those with diabetes and problems with glucose intolerance are cautioned that supplemental alpha-lipoic acid may lower blood glucose levels. Blood glucose should be monitored and antidiabetic drug dose adjusted, if necessary, to avoid possible hypoglycemia.
PDR for Nutritional Supplements 2nd ed. Thomson Reuters, Montvale, NJ 2008, p. 28
Because of lack of long-term safety data, alpha-lipoic acid should be avoided by pregnant and nursing mothers.
PDR for Nutritional Supplements 2nd ed. Thomson Reuters, Montvale, NJ 2008, p. 28
Antioxidants
Naturally occurring or synthetic substances that inhibit or retard oxidation reactions. They counteract the damaging effects of oxidation in animal tissues. (See all compounds classified as Antioxidants.)
Vitamin B Complex
A group of water-soluble vitamins, some of which are COENZYMES. (See all compounds classified as Vitamin B Complex.)
A - Alimentary tract and metabolism
A16 - Other alimentary tract and metabolism products
A16A - Other alimentary tract and metabolism products
A16AX - Various alimentary tract and metabolism products
A16AX01 - Thioctic acid
To determine the concentration of alpha-lipoic acid in the aqueous humour and investigate if its topical instillation can increase quantities. Methods: Seventy patients selected to undergo cataract surgery were randomly divided into two groups. Group 1 was used as a control group; for the patients in Group 2, a single instillation of alpha-lipoic acid eye drops (1%) was administered. Immediately before surgery an aliquot of 40-120 microL of aqueous humour was aspirated. The individual aspirations were combined to constitute pools representing time intervals with respect to administration. The levels of alpha-lipoic acid in the aqueous humour were measured using gas chromatography/mass-spectrometry. Pool 0 was created by combining the samples of aqueous humour obtained from the patients in Group 1, the control group, and the level of alpha-lipoic acid was 27.5 + 2.6 ng/mL; in the other pools the time interval between the administration of the eye drops and sampling was respectively 23 minutes, 53 minutes, 72 minutes, 93 minutes and 114 minutes, and the level of alpha-lipoic acid was 33.0 + 10.8 ng/mL; 52.0 + 2.5 ng/mL; 86.7 + 2.5 ng/mL; 91.2 + 2.5 ng/mL; 80.3 + 2.5 ng/mL. /The/ study demonstrates the presence of alpha-lipoic acid in the aqueous humour and indicates that its concentration increases after it is administered in the form of eye drops, reaching maximum values after around 93 minutes. The concentrations that are achieved in the anterior chamber allow us to theorise the possibility of exploiting the antioxidant properties of alpha-lipoic acid.
PMID:20456443 Cagini C et al; Clin Experiment Ophthalmol 38 (6): 572-6 (2010)
R(+)-alpha-lipoic acid is a natural occurring compound that acts as an essential cofactor for certain dehydrogenase complexes. The redox couple alpha-lipoic acid/dihydrolipoic acid possesses potent antioxidant activity. Exogenous racemic alpha-lipoic acid orally administered for the symptomatic treatment of diabetic polyneuropathy is readily and nearly completely absorbed, with a limited absolute bioavailability of about 30% caused by high hepatic extraction. Although the pharmacokinetics of the parent drug have been well characterized in humans, relatively little is known regarding the excretion of alpha-lipoic acid and the pharmacokinetics of any metabolites in humans. In the present study, plasma concentration-time courses, urinary excreted amounts, and pharmacokinetic parameters of alpha-lipoic acid metabolites were evaluated in 9 healthy volunteers after multiple once-daily oral administration of 600 mg racemic alpha-lipoic acid. The primary metabolic pathways of alpha-lipoic acid in man, S-methylation and beta-oxidation, were quantitatively confirmed by an HPLC-electrochemical assay newly established prior to the beginning of this study. Major circulating metabolites were the S-methylated beta-oxidation products 4,6-bismethylthio-hexanoic acid and 2,4-bismethylthio-butanoic acid, whereas its conjugated forms accounted for the major portion excreted in urine. There was no statistically significant difference in the pharmacokinetic parameters Cmax, AUC, and tmax between day 1 and day 4. Despite the prolonged half-lives of the major metabolites compared to the parent drug, no evidence of accumulation was found. Mean values of 12.4% of the administered dose were recovered in the urine after 24 hours as the sum of alpha-lipoic acid and its metabolites. The results of the present study revealed that urinary excretion of alpha-lipoic acid and five of its main metabolites does not play a significant role in the elimination of alpha-lipoic acid. Therefore, biliary excretion, further electrochemically inactive degradation products, and complete utilization of alpha-lipoic acid as a primary substrate in the endogenous metabolism should be considered.
PMID:14551180 Teichert J et al; J Clin Pharmacol 43 (11):1257-67 (2003)
In an open-label, parallel-group study involving 16 patients (8 with severely reduced renal function, 8 with end-stage renal disease needing hemodialysis), the effect of renal function on the pharmacokinetics, metabolism, and safety `of alpha-lipoic acid (thioctic acid) was evaluated by comparing the pharmacokinetic parameters with those of a reference group of 8 healthy subjects. Alpha-lipoic acid 600 mg was administered orally once daily for 4 days, and the pharmacokinetic parameters were measured on days 1 and 4. The mean percentage of the administered dose excreted in urine as parent compound was 0.2 and 0.05 in healthy subjects and subjects with severely reduced renal function, respectively. Assuming a bioavailability of 30%, this represents 0.67% and 0.17% of the bioavailable amount of alpha-lipoic acid, respectively. The percentage of total urinary recovered amounts of alpha-lipoic acid and 5 of its metabolites was 12.0 on both days. The respective values for patients with severe kidney damage were 5.2% (day 1) and 6.4% (day 4). The total percentage of the administered dose removed by hemodialysis was 4.0 in patients with end-stage renal disease. Renal clearance of alpha-lipoic acid and its major metabolites, 6,8-bismethylthio-octanoic acid, 4,6-bismethylthio-hexanoic acid and 2,4-bismethylthio-butanoic acid, were significantly decreased in subjects with kidney damage compared to the reference group. Apparent total clearance of alpha-lipoic acid was poorly correlated with creatinine clearance. There is strong evidence that alpha-lipoic acid is mainly excreted by nonrenal mechanism or further degraded to smaller units in the catabolic process. The significantly increased area under the curve values of 4,6-bismethylthio-hexanoic acid and half-lives of 2,4-bismethylthio-butanoic acid on both days in patients with severely reduced function and end-stage renal disease were not considered to be clinically relevant. Although trough levels of both metabolites tend to increase slightly in these subjects, no accumulation effects were detected. We conclude that the pharmacokinetics of alpha-lipoic acid are not influenced by creatinine clearance and are unaffected in subjects with severely reduced kidney function or end-stage renal disease. Hemodialysis did not significantly contribute to the clearance of alpha-lipoic acid. Hence, dose adjustment of alpha-lipoic acid is not necessary in patients with renal dysfunction.
PMID:15703366 Teichert J et al; J Clin Pharmacol 45 (3): 313-28 (2005)
Alpha-lipoic aicd is absorbed from the small intestine and distributed to the liver via the portal circulation and to various tissues in the body via the systemic circulation.The natural R-enantiomer is more readily absorbed than the L-enantiomer and is the more active form. Alpha-lipoic acid readily crosses the blood-brain barrier. It is found, after its distribution to the various body tissues, intracellularly, intramitochondrialy and extracellularly.
PDR for Nutritional Supplements 2nd ed. Thomson Reuters, Montvale, NJ 2008, p. 26
Alpha-lipoic acid is metabolized to its reduced form, dihydrolipoic acid by mitochondrial lipoamide dehydrogenase. Dihydroipoic acid, together with lipoic acid, form a redox couple. It is also metabolized to lipoamide, which functions as the lipoic acid cofactor in the multienzyme complexes that catalyze the oxidative decarboxylations of pyruvate and alpha-ketoglutarate. Alpha-lipoic acid may be metabolized to dithiol octanoic acid, which can undergo catabolism.
PDR for Nutritional Supplements 2nd ed. Thomson Reuters, Montvale, NJ 2008, p. 26
The excretion and biotransformation of rac-alpha-lipoic acid (LA), which is used for the symptomatic treatment of diabetic polyneuropathy, were investigated following single oral dosing of [(14)C]LA to mice (30 mg/kg), rats (30 mg/kg), dogs (10 mg/kg), and unlabeled LA to humans (600 mg). More than 80% of the radioactivity given was renally excreted. Metabolite profiles obtained by radiometric high-performance liquid chromatography revealed that LA was extensively metabolized irrespective of the species. Based on a new on-line liquid chromatography/tandem mass spectroscopy assay developed for negative ions, LA and a total of 12 metabolites were identified. Mitochondrial beta-oxidation played the paramount role in the metabolism of LA. Simultaneously, the circulating metabolites were subjected to reduction of the 1,2-dithiolane ring and subsequent S-methylation. In addition, evidence is given for the first time that the methyl sulfides formed were partly oxidized to give sulfoxides, predominantly in dogs. The disulfoxide of 2,4-bismethylmercapto-butanoic acid, the most polar metabolite identified, was the major metabolite in dogs. Furthermore, new data are presented that suggest conjugation with glycine occurred as a separate metabolic pathway in competition with beta-oxidation, predominantly in mice.
PMID:11353754 Schupke H et al; Drug Metab Dispos 29 (6): 855-62 (2001)
Alpha-lipoic acid (LA) shows a protective effect on oxidative stress-induced apoptosis while it induces apoptosis in various cancer cells. Intracellular Ca(2+) plays a central role in triggering apoptotic pathways. In the present study, we aim to investigate whether LA induces apoptosis in lung cancer cells and whether Ca(2+) is involved in LA-induced apoptosis. We found that LA decreased cell viability and increased DNA fragmentation of the cells. LA activated the caspase-independent pathway, determined by upregulation of poly(ADP-ribose) polymerase (PARP) and increased the nuclear level of apoptosis-inducing factor and caspase-dependent apoptotic pathway, determined by increased levels of cytochrome c and PARP-1 cleavage product. LA-induced apoptotic alterations were inhibited in the cells treated with Ca(2+) chelator BAPTA-AM. In conclusion, LA induces apoptosis through caspase-independent and caspase-dependent pathways, which is mediated by intracellular Ca(2+).
PMID:19723049 Choi SY et al; Ann N Y Acad Sc 1171: 149-55 (2009)
Alpha-lipoic acid is known to increase insulin sensitivity in vivo and to stimulate glucose uptake into adipose and muscle cells in vitro. In this study, alpha-lipoic acid was demonstrated to stimulate the autophosphorylation of insulin receptor and glucose uptake into 3T3-L1 adipocytes by reducing the thiol reactivity of intracellular proteins. To elucidate mechanism of this effect, role of protein thiol groups and H(2)O(2) in insulin receptor autophosphorylation and glucose uptake was investigated in 3T3-L1 adipocytes following stimulation with alpha-lipoic acid. Alpha-lipoic acid or insulin treatment of adipocytes increased intracellular level of oxidants, decreased thiol reactivity of the insulin receptor beta-subunit, increased tyrosine phosphorylation of the insulin receptor, and enhanced glucose uptake. Alpha-lipoic acid or insulin-stimulated glucose uptake was inhibited (i) by alkylation of intracellular, but not extracellular, thiol groups downstream of insulin receptor activation, and (ii) by diphenylene iodonium at the level of the insulin receptor autophosphorylation. alpha-Lipoic acid also inhibited protein tyrosine phosphatase activity and decreased thiol reactivity of protein tyrosine phosphatase 1B. These findings indicate that oxidants produced by alpha-lipoic acid or insulin are involved in activation of insulin receptor and in inactivation of protein tyrosine phosphatases, which eventually result in elevated glucose uptake into 3T3-L1 adipocytes.
PMID:12948866 Cho KJ et al; Biochem Pharmacol 66 (5): 849-58 (2003)
Reactive oxygen (ROS) and nitrogen oxide (RNOS) species are produced as by-products of oxidative metabolism. A major function for ROS and RNOS is immunological host defense. Recent evidence indicate that ROS and RNOS may also function as signaling molecules. However, high levels of ROS and RNOS have been considered to potentially damage cellular macromolecules and have been implicated in the pathogenesis and progression of various chronic diseases. alpha-Lipoic acid and dihydrolipoic acid exhibit direct free radical scavenging properties and as a redox couple, with a low redox potential of -0.32 V, is a strong reductant. Several studies provided evidence that alpha-lipoic acid supplementation decreases oxidative stress and restores reduced levels of other antioxidants in vivo. However, there is also evidence indicating that alpha-lipoic acid and dihydrolipoic acid may exert prooxidant properties in vitro. alpha-Lipoic acid and dihydrolipoic acid were shown to promote the mitochondrial permeability transition in permeabilized hepatocytes and isolated rat liver mitochondria. Dihydrolipoic acid also stimulated superoxide anion production in rat liver mitochondria and submitochondrial particles. alpha-Lipoic acid was recently shown to stimulate glucose uptake into 3T3-L1 adipocytes by increasing intracellular oxidant levels and/or facilitating insulin receptor autophosphorylation presumably by oxidation of critical thiol groups present in the insulin receptor beta-subunit. Whether alpha-lipoic acid and/or dihydrolipoic acid-induced oxidative protein modifications contribute to their versatile effects observed in vivo warrants further investigation.
PMID:12127266 Moini H et al; Toxicol Appl Pharmacol 182 (1): 84-90 (2002)
This study investigated the effect of alpha-lipoic acid (ALA) in concentration range 0.7-5.0 mM on the intracellular level of reduced glutathione, the cell cycle phase distribution, the structure of microfilaments and microtubules of normal (3T3) and transformed (3T3-SV40) fibroblasts. We obtained that ALA increased the glutathione content in transformed cells, but did not change its level in normal cells, induced cell cycle arrest of 3T3 cells (but not 3T3-SV40 cells), and disrupted actin microfilaments in cells of both lines. The effect of ALA was compared with N-acetylcysteine (NAC) action. The whole complex of findings allows us to affirm that each of these antioxidants acts on its own target molecules in normal and transformed cells and activates different signal and metabolic pathways in these cells. But at the same time the intermediate steps of ALA and NAC action can be common (alteration of the intracellular level of glutathione, reorganization of actin cytoskeleton, etc.).
PMID:20141032 Vakhromova EA et al; Tsitologiia 51 (12): 971-7 (2009)
For more Mechanism of Action (Complete) data for alpha-Lipoic acid (7 total), please visit the HSDB record page.






