



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
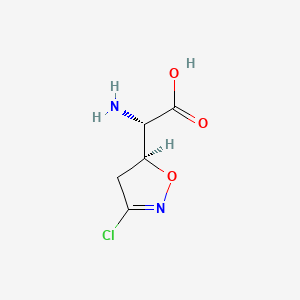

1. Alpha-amino-3-chloro-4,5-dihydro-5-isoxazoleacetic Acid
2. Alpha-s,5s-alpha-amino-3-chloro-2-isoxazoline-5-acetic Acid
3. At 125
4. At-125
5. L-(alphas,5s)-alpha-amino-3-chloro-4,5-dihydro-5-isoxazoleacetic Acid
6. Nsc 163501
7. U 42,126
8. U-42,126
1. 42228-92-2
2. Antibiotic At 125
3. At-125
4. Acivicine
5. At 125
6. Acivicino
7. Acivicinum
8. Nsc-163501
9. Acia
10. Nsc 163501
11. U 42126
12. U-42,126
13. Nsc163501
14. (2s)-2-amino-2-[(5s)-3-chloro-4,5-dihydro-1,2-oxazol-5-yl]acetic Acid
15. U-42126
16. U 42,126
17. (alphas,5s)-alpha-amino-3-chloro-2-isoxazoline-5-acetic Acid
18. Chebi:74545
19. (s)-2-amino-2-((s)-3-chloro-4,5-dihydroisoxazol-5-yl)acetic Acid
20. O0x60k76i6
21. (alpha-s,5s)-alpha-amino-3-chloro-4,5-dihydro-5-isoxazoleacetic Acid
22. (2s)-amino[(5s)-3-chloro-4,5-dihydro-1,2-oxazol-5-yl]ethanoic Acid
23. Acivicin [usan:inn]
24. Acivicine [inn-french]
25. Acivicinum [inn-latin]
26. Acivicino [inn-spanish]
27. Unii-o0x60k76i6
28. Ncgc00095996-01
29. Brn 1074648
30. Spectrum_000343
31. Acivicin [usan]
32. Acivicin (usan/inn)
33. Acivicin [inn]
34. Spectrum3_001857
35. Spectrum4_000902
36. Spectrum5_001706
37. Acivicin [mart.]
38. Acivicin [who-dd]
39. Ncimech_000028
40. Schembl4159
41. Dsstox_cid_26010
42. Dsstox_rid_81286
43. Dsstox_gsid_46010
44. Bspbio_003274
45. Kbiogr_001364
46. Kbioss_000823
47. Divk1c_000672
48. Spectrum1502002
49. Acivicin, >=98% (hplc)
50. Chembl1231101
51. Dtxsid0046010
52. Gtpl11402
53. Hms502b14
54. Kbio1_000672
55. Kbio2_000823
56. Kbio2_003391
57. Kbio2_005959
58. Kbio3_002775
59. Ninds_000672
60. At125
61. Zinc3871381
62. Tox21_111545
63. (2s)-amino[(5s)-3-chloro-4,5-dihydro-1,2-oxazol-5-yl]acetic Acid
64. (alphas,5s)-alpha-amino-3-chlor-4,5-dihydro-5-isoxazolylessigsaeure
65. (s-(r*,r*))-4,5-dihydro-alpha-amino-3-chloro-5-isoxazoleacetic Acid
66. Ccg-35436
67. Mfcd00866432
68. Akos027327575
69. Cs-w017302
70. Hy-w016586
71. Idi1_000672
72. Ncgc00178172-01
73. As-56028
74. Nci60_001241
75. Cas-42228-92-2
76. D02755
77. A825818
78. Q4674217
79. 5-isoxazoleacetic Acid,5-dihydro-, [s-(r*,r*)]-
80. Brd-k11632748-001-02-5
81. Alphas-amino-3-chloro-4,5s-dihydro-5-isoxazoleacetic Acid
82. (2s)-amino[(5s)-3-chloro-4,5-dihydroisoxazol-5-yl]ethanoic Acid
83. (s)-2-amino-2-((s)-3-chloro-4,5-dihydroisoxazol-5-yl)aceticacid
84. (.alpha.s,5s)-.alpha.-amino-3-chloro-2-isoxazoline-5-acetic Acid
85. (2s)-2-amino-2-[(5s)-3-chloro-4,5-dihydroisoxazol-5-yl]acetic Acid
86. (alphas,5s)-alpha-amino-3-chloro- 4,5-dihydro-5-isoxazol-acetic Acid
87. (alphas,5s)-alpha-amino-3-chloro-4,5-dihydro-5-isoxazol-acetic Acid
88. (alphas,5s)-alpha-amino-3-chloro-4,5-dihydro-5-isoxazole Acetic Acid
89. (alphas,5s)-alpha-amino-3-chloro-4,5-dihydro-5-isoxazoleacetic Acid
90. .alpha.-amino-3-chloro-4,5-dihydro-[s-(r*,r*)]-5-isoxazoleacetatic Acid
91. 5-isoxazoleacetic Acid, Alpha-amino-3-chloro-4,5-dihydro-, (s-(r*,r*))-
92. 5-isoxazoleacetic Acid, .alpha.-amino-3-chloro-4,5-dihydro-, (s-(r*,r*))-
93. 5-isoxazoleacetic Acid, 4,5-dihydro-alpha-amino-3-chloro-, (s-(r*,r*))-
| Molecular Weight | 178.57 g/mol |
|---|---|
| Molecular Formula | C5H7ClN2O3 |
| XLogP3 | -2.7 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 2 |
| Exact Mass | 178.0145198 g/mol |
| Monoisotopic Mass | 178.0145198 g/mol |
| Topological Polar Surface Area | 84.9 Ų |
| Heavy Atom Count | 11 |
| Formal Charge | 0 |
| Complexity | 206 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Antimetabolites
Drugs that are chemically similar to naturally occurring metabolites, but differ enough to interfere with normal metabolic pathways. (From AMA Drug Evaluations Annual, 1994, p2033) (See all compounds classified as Antimetabolites.)
Antibiotics, Antineoplastic
Chemical substances, produced by microorganisms, inhibiting or preventing the proliferation of neoplasms. (See all compounds classified as Antibiotics, Antineoplastic.)
Antifungal Agents
Substances that destroy fungi by suppressing their ability to grow or reproduce. They differ from FUNGICIDES, INDUSTRIAL because they defend against fungi present in human or animal tissues. (See all compounds classified as Antifungal Agents.)
Enzyme Inhibitors
Compounds or agents that combine with an enzyme in such a manner as to prevent the normal substrate-enzyme combination and the catalytic reaction. (See all compounds classified as Enzyme Inhibitors.)
Antimetabolites, Antineoplastic
Antimetabolites that are useful in cancer chemotherapy. (See all compounds classified as Antimetabolites, Antineoplastic.)


