


API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
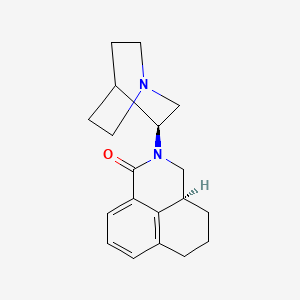

1. 2-(1-azabicyclo(2.2.2)oct-3-yl)-2,3,3a,4,5,6-hexahydro-1h-benz(de)isoquinolin-1-one
2. 2-qhbiqo
3. Aloxi
4. Palonosetron Hydrochloride
5. Palonosetron, (3r)-
6. Palonosetron, (r-(r*,r*))-isomer
7. Palonosetron, (r-(r*,s*))-isomer
8. Palonosetron, (s-(r*,s*))-isomer
9. Rs 25233 197
10. Rs 25233 198
11. Rs 25233-197
12. Rs 25233-198
13. Rs 25233197
14. Rs 25233198
15. Rs 25259
16. Rs 25259 197
17. Rs 25259 198
18. Rs 25259-197
19. Rs 25259-198
20. Rs 25259197
21. Rs 25259198
22. Rs-25233-197
23. Rs-25233-198
24. Rs-25259
25. Rs-25259-197
26. Rs-25259-198
27. Rs25233197
28. Rs25233198
29. Rs25259
30. Rs25259197
31. Rs25259198
1. 135729-61-2
2. (-)-palonosetron
3. Aloxi
4. Palonosetron [inn]
5. Aloxi (tn)
6. Palonosetron, (3as, 3s)-
7. (3as)-2-[(3s)-1-azabicyclo[2.2.2]octan-3-yl]-3a,4,5,6-tetrahydro-3h-benzo[de]isoquinolin-1-one
8. 2-qhbiqo
9. Chebi:85161
10. Palonosetron (inn)
11. 2-(1-azabicyclo(2.2.2)oct-3-yl)-2,3,3a,4,5,6-hexahydro-1h-benz(de)isoquinolin-1-one
12. 5d06587d6r
13. Nsc-743769
14. (3~{a}~{s})-2-[(3~{s})-1-azabicyclo[2.2.2]octan-3-yl]-3~{a},4,5,6-tetrahydro-3~{h}-benzo[de]isoquinolin-1-one
15. (3as)-2-[(3s)-1-azabicyclo[2.2.2]octan-3-yl]-2,3,3a,4,5,6-hexahydro-1h-benzo[de]isoquinolin-1-one
16. Palonosetronum
17. Unii-5d06587d6r
18. 149653-99-6
19. Palonosetron; Aloxi
20. Palonosetron [mi]
21. Schembl3746
22. Palonosetron [vandf]
23. (s-(r*,r*))-2-(1-azabicyclo(2.2.2)oct-3-yl)-2,3,3a,4,5,6-hexahydro-1h-benz(de)isoquinolin-1-one
24. Palonosetron [who-dd]
25. Gtpl7486
26. Chembl1189679
27. Dtxsid5048342
28. Schembl13391549
29. (s)-2-((s)-quinuclidin-3-yl)-2,3,3a,4,5,6-hexahydro-1h-benzo[de]isoquinolin-1-one
30. Hms3886a22
31. Bcp07225
32. Hy-a0018
33. Zinc3795819
34. Bdbm50417287
35. Mfcd07783848
36. S5740
37. Akos015967749
38. Akos025311243
39. Cs-0385
40. Db00377
41. Nsc 743769
42. Ncgc00271490-05
43. As-35217
44. P-226
45. D07175
46. Ab00698542-05
47. Ab00698542_07
48. 653p996
49. Q-100993
50. Z2216208607
51. (3as)-2-[(3s)-1-azabicyclo[2.2.2]octan-3-yl]-3a,4,5,6-tetrahydro-3h-benzo[de]isoquinolin-1-one.
52. (5s)-3-[(3s)-1-azabicyclo[2.2.2]octan-3-yl]-3-azatricyclo[7.3.1.0^{5,13}]trideca-1(12),9(13),10-trien-2-one
53. 1021481-16-2
54. 1h-benz(de)isoquinolin-1-one, 2-(1-azabicyclo(2.2.2)oct-3-yl)-2,3,3a,4,5,6-hexahydro-, (s-(r*,r*))-
55. 1h-benz(de)isoquinolin-1-one, 2-(3s)-1-azabicyclo(2.2.2)oct-3-yl-2,3,3a,4,5,6-hexahydro-, (3as)-
| Molecular Weight | 296.4 g/mol |
|---|---|
| Molecular Formula | C19H24N2O |
| XLogP3 | 2.8 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 1 |
| Exact Mass | 296.188863393 g/mol |
| Monoisotopic Mass | 296.188863393 g/mol |
| Topological Polar Surface Area | 23.6 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 456 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For the prevention of acute and delayed nausea and vomiting associated with initial and repeat courses of moderately emetogenic cancer chemotherapy, as well as prevention of acute nausea and vomiting associated with highly emetogenic cancer chemotherapy. Also used for the prevention of postoperative nausea and vomiting for up to 24 hours post operation.
FDA Label
Palonosetron Accord is indicated in adults for:
- the prevention of acute nausea and vomiting associated with highly emetogenic cancer chemotherapy,
- the prevention of nausea and vomiting associated with moderately emetogenic cancer chemotherapy.
Palonosetron Accord is indicated in paediatric patients 1 month of age and older for:
- The prevention of acute nausea and vomiting associated with highly emetogenic cancer chemotherapy and prevention of nausea and vomiting associated with moderately emetogenic cancer chemotherapy.
Palonosetron Hospira is indicated in adults for:
- the prevention of acute nausea and vomiting associated with highly emetogenic cancer chemotherapy;
- the prevention of nausea and vomiting associated with moderately emetogenic cancer chemotherapy.
Palonosetron Hospira is indicated in paediatric patients 1 month of age and older for:
- the prevention of acute nausea and vomiting associated with highly emetogenic cancer chemotherapy and prevention of nausea and vomiting associated with moderately emetogenic cancer chemotherapy.
Aloxi is indicated in adults for:
- the prevention of acute nausea and vomiting associated with highly emetogenic cancer chemotherapy,
- the prevention of nausea and vomiting associated with moderately emetogenic cancer chemotherapy.
Aloxi is indicated in paediatric patients 1 month of age and older for:
- the prevention of acute nausea and vomiting associated with highly emetogenic cancer chemotherapy and prevention of nausea and vomiting associated with moderately emetogenic cancer chemotherapy.
Palonosetron is an antinauseant and antiemetic agent indicated for the prevention of nausea and vomiting associated with moderately-emetogenic cancer chemotherapy and for the prevention of postoperative nausea and vomiting. Palonosetron is a highly specific and selective serotonin 5-HT3 receptor antagonist that is pharmacologically related to other 5-HT3 receptor antagonists, but differs structurally. Palonosetron has a high affinity for 5-HT3 receptors, but has little to no affinity for other receptors. The serontonin 5-HT3 receptors are located on the nerve terminals of the vagus in the periphery, and centrally in the chemoreceptor trigger zone of the area postrema. It is suggested that chemotherapeutic agents release serotonin from the enterochromaffin cells of the small intestine by causing degenerative changes in the GI tract. The serotonin then stimulates the vagal and splanchnic nerve receptors that project to the medullary vomiting center, as well as the 5-HT3 receptors in the area postrema, thus initiating the vomiting reflex, causing nausea and vomiting.
Antiemetics
Drugs used to prevent NAUSEA or VOMITING. (See all compounds classified as Antiemetics.)
Serotonin 5-HT3 Receptor Antagonists
Drugs that bind to but do not activate SEROTONIN 5-HT3 RECEPTORS, thereby blocking the actions of SEROTONIN or SEROTONIN 5-HT3 RECEPTOR AGONISTS. (See all compounds classified as Serotonin 5-HT3 Receptor Antagonists.)
A04AA05
A04AA05
A04AA05
A04AA05
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
A - Alimentary tract and metabolism
A04 - Antiemetics and antinauseants
A04A - Antiemetics and antinauseants
A04AA - Serotonin (5ht3) antagonists
A04AA05 - Palonosetron
Absorption
Low oral bioavailability.
Route of Elimination
After a single intravenous dose of 10 mcg/kg [14C]-palonosetron, approximately 80% of the dose was recovered within 144 hours in the urine
Volume of Distribution
8.3 2.5 L/kg
Clearance
160 +/- 35 mL/h/kg
Hepatic (50%), primarily CYP2D6-mediated, although CYP3A4 and CYP1A2 are also involved.
Approximately 40 hours
Palonosetron is a selective serotonin 5-HT3 receptor antagonist. The antiemetic activity of the drug is brought about through the inhibition of 5-HT3 receptors present both centrally (medullary chemoreceptor zone) and peripherally (GI tract). This inhibition of 5-HT3 receptors in turn inhibits the visceral afferent stimulation of the vomiting center, likely indirectly at the level of the area postrema, as well as through direct inhibition of serotonin activity within the area postrema and the chemoreceptor trigger zone. Alternative mechanisms appear to be primarily responsible for delayed nausea and vomiting induced by emetogenic chemotherapy, since similar temporal relationships between between serotonin and emesis beyond the first day after a dose have not been established, and 5-HT3 receptor antagonists generally have not appeared to be effective alone in preventing or ameliorating delayed effects. It has been hypothesized that palonosetron's potency and long plasma half-life may contribute to its observed efficacy in preventing delayed nausea and vomiting caused by moderately emetogenic cancer chemotherapy.


