



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
Other Suppliers
0

USA (Orange Book)

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
Annual Reports
0
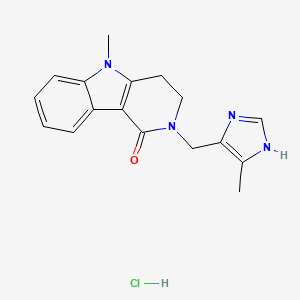

1. 2,3,4,5-tetrahydro-5-methyl-2-((5-methylimidazol-4-yl)methyl)-1h-pyrido(4,3-b)indol-1-one Monohydrochloride
2. Alosetron
3. Alosetron Monohydrochloride
4. Gr 68755
5. Gr68755
6. Lotronex
1. 122852-69-1
2. Alosetron Hcl
3. Lotronex
4. Gr 68755c
5. Alosetron (hydrochloride)
6. Alosetron Hydrochloride(1:x)
7. Lotrpnex
8. Alosetron Hydrochloride [usan]
9. 132414-02-9
10. 5-methyl-2-[(5-methyl-1h-imidazol-4-yl)methyl]-3,4-dihydropyrido[4,3-b]indol-1-one Hydrochloride
11. Gr 68755
12. Alosetron (hydrochloride(1:x))
13. 5-methyl-2-[(5-methyl-1h-imidazol-4-yl)methyl]-3,4-dihydropyrido[4,3-b]indol-1-one;hydrochloride
14. Gr 68755x
15. Gr-68755c
16. 2f5r1a46yw
17. 1h-pyrido[4,3-b]indol-1-one, 2,3,4,5-tetrahydro-5-methyl-2-[(4-methyl-1h-imidazol-5-yl)methyl]-, Hydrochloride (1:1)
18. 2,3,4,5-tetrahydro-5-methyl-2-[(5-methyl-1h-imidazol-4-yl)methyl]-1h-pyrido[4,3-b]indol-1-one Hydrochloride
19. Gr 68755;gr 68755x
20. 122852-69-1 (hcl)
21. Dsstox_cid_24208
22. Dsstox_rid_80120
23. Dsstox_gsid_44208
24. 1h-pyrido(4,3-b)indol-1-one, 2,3,4,5-tetrahydro-5-methyl-2-((5-methyl-1h-imidazol-4-yl)methyl)-, Monohydrochloride
25. 2,3,4,5-tetrahydro-5-methyl-2-((5-methylimidazol-4-yl)methyl)-1h-pyrido(4,3-b)indol-1-one Monohydrochloride
26. Alosetron Hydrochloride (usan)
27. Mls001401464
28. Gr 68755 (hydrochloride(1:x));gr 68755x (hydrochloride(1:x))
29. Chebi:53783
30. Cas-122852-69-1
31. Hsdb 7055
32. Ncgc00167528-01
33. Smr000469211
34. Unii-2f5r1a46yw
35. Lotrpnex (tn)
36. 5-methyl-2-((5-methyl-1h-imidazol-4-yl)methyl)-2,3,4,5-tetrahydro-1h-pyrido[4,3-b]indol-1-one Hydrochloride
37. 5-methyl-2-[(5-methyl-1h-imidazol-4-yl)methyl]-2,3,4,5-tetrahydro-1h-pyrido[4,3-b]indol-1-one Hydrochloride
38. Schembl806
39. Alosetron Hcl [vandf]
40. Regid_for_cid_60758
41. Alosetron Hydrochloride- Bio-x
42. Chembl1200885
43. Dtxsid8044208
44. Amy33432
45. Bcp08834
46. Alosetron Hydrochloride [mi]
47. Tox21_112525
48. Ac-022
49. Hy-70050c
50. Mfcd03453647
51. S4694
52. Alosetron Hydrochloride [hsdb]
53. Akos015889472
54. Akos015961658
55. Tox21_112525_1
56. Alosetron Hydrochloride [mart.]
57. Ccg-100910
58. Ccg-267816
59. Cs-0642
60. Nc00160
61. Alosetron Hydrochloride [usp-rs]
62. Alosetron Hydrochloride [who-dd]
63. Ncgc00167528-02
64. 1h-pyrido(4,3-b)indol-1-one, 2,3,4,5-tetrahydro-5-methyl-2((5-methyl-1h-imidazol-4-yl)methyl)-, Monohydrochloride
65. Alosetron Hydrochloride, >=98% (hplc)
66. Ba166465
67. Bs-42103
68. Gr-68755
69. Alosetron Hydrochloride [orange Book]
70. Ft-0631109
71. Ft-0661526
72. A14989
73. Alosetron Hydrochloride [usp Monograph]
74. D02829
75. D88551
76. 852a691
77. A804978
78. Q-200613
79. Q27124209
80. Gr 68755c; Gr 68755; Gr 68755x;gr68755c; Gr68755; Gr68755x
81. 2,3,4,5-tetrahydro-5-methyl-2-[(5-methyl-1-h-imidazol-4-yl)methyl]-1h-pyrido[4,3-b]indol-1-one Hydrochloride
82. 5-methyl-2-((5-methyl-1h-imidazol-4-yl)methyl)-3,4-dihydro-2h-pyrido[4,3-b]indol-1(5h)-one Hydrochloride
| Molecular Weight | 330.8 g/mol |
|---|---|
| Molecular Formula | C17H19ClN4O |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 2 |
| Exact Mass | 330.1247389 g/mol |
| Monoisotopic Mass | 330.1247389 g/mol |
| Topological Polar Surface Area | 53.9 Ų |
| Heavy Atom Count | 23 |
| Formal Charge | 0 |
| Complexity | 442 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 2 | |
|---|---|
| Drug Name | Lotronex |
| PubMed Health | Alosetron (By mouth) |
| Drug Classes | Antidiarrheal, Gastrointestinal Agent |
| Drug Label | The active ingredient in LOTRONEX Tablets is alosetron hydrochloride (HCl), a potent and selective antagonist of the serotonin 5-HT3 receptor type. Chemically, alosetron is designated as 2,3,4,5-tetrahydro-5-methyl-2-[(5-methyl-1H-imidazol-4-yl)methy... |
| Active Ingredient | Alosetron hydrochloride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 0.5mg base; eq 1mg base |
| Market Status | Prescription |
| Company | Prometheus Labs |
| 2 of 2 | |
|---|---|
| Drug Name | Lotronex |
| PubMed Health | Alosetron (By mouth) |
| Drug Classes | Antidiarrheal, Gastrointestinal Agent |
| Drug Label | The active ingredient in LOTRONEX Tablets is alosetron hydrochloride (HCl), a potent and selective antagonist of the serotonin 5-HT3 receptor type. Chemically, alosetron is designated as 2,3,4,5-tetrahydro-5-methyl-2-[(5-methyl-1H-imidazol-4-yl)methy... |
| Active Ingredient | Alosetron hydrochloride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 0.5mg base; eq 1mg base |
| Market Status | Prescription |
| Company | Prometheus Labs |
Alosetron, a selective 5-HT3-receptor antagonist, is indicated for the treatment of irritable bowel syndrome in female patients whose predominant symptom is diarrhea. /Salt not specified/
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1041
... Animal models have shown it to be active in anxiety, psychosis, cognitive impairment, emesis & drug withdrawal, though its application in humans has been almost entirely restricted to irritable bowel syndrome (IBS). ... Alosetron appears promising in the treatment of abdominal pain & discomfort & normalising of bowel function in patients with non-constipated IBS. It also improves quality of life, has a high degree of tolerability & has an excellent safety profile to date. /Salt not specified/
PMID:11060667 Camilleri M; Expert Opin Investig Drugs 9(1): 147-159 (2000)
Plasma concentrations of alosetron are 30 to 50% lower in men than in women given the same oral dose. In patients with irritable bowel syndrome, concentrations of alosetron are influenced by gender. Efficacy has not been established in men at any dose. /Salt not specified/
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional 21 st ed. Volume 1. MICROMEDEX Thomson Health Care, Englewood, CO. 2001. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 58
/Alosetron/ should not be used in irritable bowel syndrome patients currently constipated or whose predominant bowel symptom in constipation because constipation is a frequent side effect of alosetron. /Salt not specified/
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional 21 st ed. Volume 1. MICROMEDEX Thomson Health Care, Englewood, CO. 2001. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 58
... Constipation is the most frequent adverse event, with a higher incidence of transient constipation in alosetron-treated patients, typically occurring in the first month of treatment. /Salt not specified/
PMID:11280555 Wolfe SG, et al; Am J Gastroenterol 96(3): 803-811 (2001)
Significant side effects have been noted with the use of alosetron including severe constipation, fecal impaction, & ischemic colitis. ...A case of ischemic colitis in a male patient with IBS who was briefly treated with alosetron /is described/. Clinical, endoscopic, & pathologic features of the focal colitis strongly suggested ischemia. Symptoms correlated temporally with alosetron use, & symptoms abated with discontinuation of the drug. Endoscopic & pathologic resolution of the colitis were documented. /salt not specified/
PMID:11159896 Friedel D, et al; Gastroenterology 120(2): 557-560 (2001)
Gastrointestinal Agents
Drugs used for their effects on the gastrointestinal system, as to control gastric acidity, regulate gastrointestinal motility and water flow, and improve digestion. (See all compounds classified as Gastrointestinal Agents.)
Serotonin Antagonists
Drugs that bind to but do not activate serotonin receptors, thereby blocking the actions of serotonin or SEROTONIN RECEPTOR AGONISTS. (See all compounds classified as Serotonin Antagonists.)
Absorption is rapid and ranges from 30 to > 90% after oral administration. /Salt not specified/
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional 21 st ed. Volume 1. MICROMEDEX Thomson Health Care, Englewood, CO. 2001. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 58
Alosetron ... is absorbed rapidly after oral admin & is widely distributed throughout tissues after oral or iv. dosing in animals. Its metab is rapid & extensive with N-demethylation, hydroxylation & oxidation. The drug, or its two principal metabolites, is equally excreted through the biliary tract & kidneys. Alosetron has proved safe in a range of toxicity studies; at high repeated dosing, clinical signs were transient & repeated admin produced no significant adverse effects on fertility, reproductive performance or fetal development. In pharmacokinetic studies, bioavailability of alosetron in healthy volunteers is approx 60% & the plasma half-life is about 1.5 hr. There are some gender differences in the pharmacokinetic profile, with 30-50% higher alosetron concns in females. No consistent differences in alosetron serum concns between the young & elderly were observed. The pharmacokinetics of single, oral doses of alosetron are linear up to 8 mg. ... /Salt not specified/
PMID:11060667 Camilleri M; Expert Opin Investig Drugs 9(1): 147-159 (2000)
/Alosetron/ metab is rapid & extensive with N-demethylation, hydroxylation & oxidation. The drug, or its two principal metabolites, is equally excreted through the biliary tract & kidneys. Alosetron has proved safe in a range of toxicity studies; at high repeated dosing, clinical signs were transient & repeated admin produced no significant adverse effects on fertility, reproductive performance or fetal development. ... /Salt not specified/
PMID:11060667 Camilleri M; Expert Opin Investig Drugs 9(1): 147-159 (2000)
Alosetron is extensively metabolized by multiple cytochrome P450 (CYP) enzymes, including CYP2C9 & CYP3A4.
PMID:11304903 D'Souza DL, et al; J Clin Pharmacol 41(4): 455-458 (2001)
Plasma half life is about 1.5 hours.
PMID:11060667 Camilleri M; Expert Opin Investig Drugs 9(1): 147-159 (2000)
Serotonin 5HT3-receptor antagonist /Salt not specified/
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th Edition, Whitehouse Station, NJ: Merck and Co., Inc., 2001., p. 56
... 5-HT3 antagonists delay colonic transit, incr colonic compliance, & incr small intestinal water absorption. ... /Salt not specified/
PMID:10886042 Thumshirn M, et al; Aliment Pharmacol Ther 14(7): 869-878 (2000)
Alosetron (Lotronex) is a potent, highly selective 5-HT(3) antagonist. ... /Salt not specified/
PMID:11060667 Camilleri M; Expert Opin Investig Drugs 9(1): 147-159 (2000)






