



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
Other Suppliers

USA (Orange Book)

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
U.S. Medicaid
Annual Reports
0
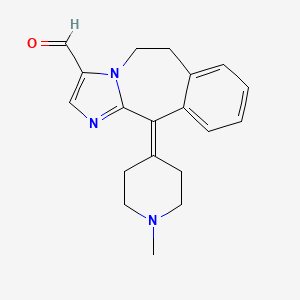

1. 147084-10-4
2. Lastacaft
3. R89674
4. R-89674
5. R 89674
6. 7z8o94ecsx
7. Chebi:71023
8. 11-(1-methylpiperidin-4-ylidene)-5,6-dihydroimidazo[2,1-b][3]benzazepine-3-carbaldehyde
9. Lastacaft (tn)
10. 11-(1-methylpiperidin-4-ylidene)-6,11-dihydro-5h-imidazo[2,1-b][3]benzazepine-3-carbaldehyde
11. 2-(1-methylpiperidin-4-ylidene)-4,7-diazatricyclo[8.4.0.0(3,7)]tetradeca- 1(14),3,5,10,12-pentaene-6-carbaldehyde
12. Alcaftadine [usan:inn]
13. Unii-7z8o94ecsx
14. Alcaftadina
15. Alcaftadinum
16. 5h-imidazo(2,1-b)(3)benzazepine-3-carboxaldehyde, 6,11-dihydro-11-(1-methyl-4-piperidinylidene)-
17. 5h-imidazo[2,1-b][3]benzazepine-3-carboxaldehyde, 6,11-dihydro-11-(1-methyl-4-piperidinylidene)-
18. Alcaftadine [inn]
19. Alcaftadine [jan]
20. Alcaftadine [usan]
21. Alcaftadine [vandf]
22. Alcaftadine [mart.]
23. Alcaftadine [who-dd]
24. Alcaftadine (jan/usan/inn)
25. Gtpl7587
26. Schembl1602418
27. Chembl1201747
28. Alcaftadine, >=98% (hplc)
29. (unlabelled)alcaftadine-13c-d3
30. Alcaftadine [orange Book]
31. Dtxsid80598455
32. Hms3885b12
33. Bcp04261
34. Mfcd09954106
35. S4625
36. Zinc11726211
37. Akos025402002
38. Am84429
39. Bcp9000269
40. Ccg-267551
41. Db06766
42. Ncgc00390732-04
43. 5h-imidazo[2,1-b][3]benzazepine-3-carboxaldehyde,6,11-dihydro-11-(1-methyl-4-piperidinylidene)-
44. Ac-28019
45. As-56303
46. Hy-17039
47. Bcp0726000082
48. Db-063693
49. Ft-0661472
50. A16393
51. D06552
52. Q4712900
53. 11-(1-methylpiperidin-4-ylidene)-5,6-dihydroimidazo[2,3-b][3]benzazepine-3-carbaldehyde
54. 11-(1-methylpiperidin-4-ylidene)-6,11-dihydro-5h-benzo[d]imidazo[1,2-a]azepine-3-carbaldehyde
55. 11-(1-methylpiperidin-4-ylidene)-6,11-dihydro-5h-imidazolo(2,1-b)(3)benzazepine-3-carbaldehyde
56. 2-(1-methylpiperidin-4-ylidene)-4,7-diazatricyclo[8.4.0.0^{3,7}]tetradeca-1(14),3,5,10,12-pentaene-6-carbaldehyde
57. 4-(1-methyl-piperidin-4-ylidene)-9,10-dihydro-4h-3,10a-diaza-benzo(f)azulene-1-carbaldehyde
58. 5h-imidazo(2,1-b)(3)benzazepine-3-carboxaldehyde, 6,11-dihydro-11-(1-methyl-4- Piperidinylidene)-
| Molecular Weight | 307.4 g/mol |
|---|---|
| Molecular Formula | C19H21N3O |
| XLogP3 | 1.7 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 1 |
| Exact Mass | 307.168462302 g/mol |
| Monoisotopic Mass | 307.168462302 g/mol |
| Topological Polar Surface Area | 38.1 Ų |
| Heavy Atom Count | 23 |
| Formal Charge | 0 |
| Complexity | 479 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Lastacaft |
| PubMed Health | Alcaftadine (Into the eye) |
| Drug Classes | Ophthalmologic Agent |
| Drug Label | LASTACAFT is a sterile, topically administered H1 receptor antagonist containing alcaftadine for ophthalmic use. Alcaftadine is a white to yellow powder with an empirical formula of C19H21N3O and a molecular weight of 307.39. Contains: Active: alca... |
| Active Ingredient | Alcaftadine |
| Dosage Form | Solution/drops |
| Route | Ophthalmic |
| Strength | 0.25% |
| Market Status | Prescription |
| Company | Allergan |
| 2 of 2 | |
|---|---|
| Drug Name | Lastacaft |
| PubMed Health | Alcaftadine (Into the eye) |
| Drug Classes | Ophthalmologic Agent |
| Drug Label | LASTACAFT is a sterile, topically administered H1 receptor antagonist containing alcaftadine for ophthalmic use. Alcaftadine is a white to yellow powder with an empirical formula of C19H21N3O and a molecular weight of 307.39. Contains: Active: alca... |
| Active Ingredient | Alcaftadine |
| Dosage Form | Solution/drops |
| Route | Ophthalmic |
| Strength | 0.25% |
| Market Status | Prescription |
| Company | Allergan |
For the prevention of itching associated with allergic conjunctivitis.
FDA Label
Following bilateral topical ocular administration of alcaftadine ophthalmic solution, 0.25%, the mean plasma Cmax of alcaftadine was approximately 60 pg/mL and the median Tmax occurred at 15 minutes. Plasma concentrations of alcaftadine were below the lower limit of quantification (10 pg/mL) by 3 hours after dosing. The mean Cmax of the active carboxylic acid metabolite was approximately 3 ng/mL and occurred at 1 hour after dosing. Plasma concentrations of the carboxylic acid metabolite were below the lower limit of quantification (100 pg/mL) by 12 hours after dosing.
Histamine H1 Antagonists
Drugs that selectively bind to but do not activate histamine H1 receptors, thereby blocking the actions of endogenous histamine. Included here are the classical antihistaminics that antagonize or prevent the action of histamine mainly in immediate hypersensitivity. They act in the bronchi, capillaries, and some other smooth muscles, and are used to prevent or allay motion sickness, seasonal rhinitis, and allergic dermatitis and to induce somnolence. The effects of blocking central nervous system H1 receptors are not as well understood. (See all compounds classified as Histamine H1 Antagonists.)
S - Sensory organs
S01 - Ophthalmologicals
S01G - Decongestants and antiallergics
S01GX - Other antiallergics
S01GX11 - Alcaftadine
Route of Elimination
Based on data following oral administration of alcaftadine, the carboxylic acid metabolite is primarily eliminated unchanged in the urine.
The metabolism of alcaftadine is mediated by non-CYP450 cytosolic enzymes to the active carboxylic acid metabolite.
The elimination half-life of the carboxylic acid metabolite is approximately 2 hours following topical ocular administration.
Alcaftadine is a H1 histamine receptor antagonist and inhibitor of the release of histamine from mast cells. Decreased chemotaxis and inhibition of eosinophil activation has also been demonstrated.


