
Synopsis
Synopsis
0
CEP/COS
0
EU WC
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
FDA Orange Book
0
Europe
0
Canada
0
Australia
0
South Africa
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
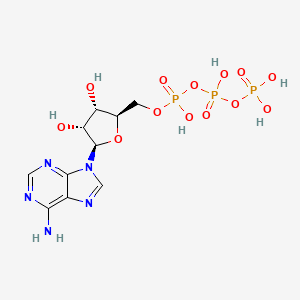

1. Adenosine Triphosphate
2. Adenosine Triphosphate, Calcium Salt
3. Adenosine Triphosphate, Chromium Ammonium Salt
4. Adenosine Triphosphate, Chromium Salt
5. Adenosine Triphosphate, Magnesium Chloride
6. Adenosine Triphosphate, Magnesium Salt
7. Adenosine Triphosphate, Manganese Salt
8. Adenylpyrophosphate
9. Atp
10. Atp Mgcl2
11. Atp-mgcl2
12. Atriphos
13. Caatp
14. Chromium Adenosine Triphosphate
15. Cr(h2o)4 Atp
16. Cratp
17. Magnesium Adenosine Triphosphate
18. Manganese Adenosine Triphosphate
19. Mgatp
20. Mnatp
21. Striadyne
1. Adenosine Triphosphate
2. 56-65-5
3. Adenosine 5'-triphosphate
4. Atp
5. Triphosphaden
6. Atipi
7. Myotriphos
8. Striadyne
9. Triadenyl
10. Atriphos
11. Adenosine 5'-(tetrahydrogen Triphosphate)
12. Glucobasin
13. Adephos
14. Adetol
15. Adynol
16. 5'-atp
17. Triphosaden
18. Adenylpyrophosphoric Acid
19. Triphosphoric Acid Adenosine Ester
20. Adenosine 5'-triphosphoric Acid
21. Atp (nucleotide)
22. Cardenosine
23. Fosfobion
24. Adenosintriphosphorsaeure
25. Ara-atp
26. Adenosine-triphosphate
27. Ado-5'-p-p-p
28. H4atp
29. 11016-17-4
30. 9-beta-d-arabinofuranosyladenine 5'-triphosphate
31. Chebi:15422
32. Adenylpyrophosphate
33. Adenosine, 5'-(tetrahydrogen Triphosphate)
34. Adenosine 5' Triphosphate
35. 3-methylbenzo[d]thiazol-3-ium Chloride
36. Chembl14249
37. 8l70q75fxe
38. 126827-79-0
39. Adenosine-5'-triphosphoric Acid
40. ((2r,3s,4r,5r)-5-(6-amino-9h-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl Tetrahydrogen Triphosphate
41. [[(2r,3s,4r,5r)-5-(6-aminopurin-9-yl)-3,4-dihydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl] Phosphono Hydrogen Phosphate
42. 119439-06-4
43. Ncgc00163309-01
44. 126339-06-8
45. Atp Disodium Salt Hydrate
46. Phosphobion
47. 51963-61-2
48. ({[({[(2r,3s,4r,5r)-5-(6-amino-9h-purin-9-yl)-3,4-dihydroxyoxolan-2-yl]methoxy}(hydroxy)phosphoryl)oxy](hydroxy)phosphoryl}oxy)phosphonic Acid
49. Einecs 200-283-2
50. Unii-8l70q75fxe
51. Mfcd00065467
52. 1kxp
53. 1mau
54. 1maw
55. 1vjc
56. 1vjd
57. 1xsc
58. 1yid
59. 2cbz
60. 2fgh
61. Adenylpyrophosphorate
62. Triphosadenine (dcf)
63. 1gz3
64. 1gz4
65. 1r0x
66. 1y8p
67. Bdbm2
68. 3h-adenosine Triphosphate
69. Dsstox_cid_2559
70. Bmse000006
71. Bmse000854
72. Bmse000993
73. Epitope Id:135612
74. Adenosine 5'-triphosphorate
75. Schembl8979
76. Dsstox_rid_76629
77. Dsstox_gsid_22559
78. Gtpl1713
79. Dtxsid6022559
80. 1b38
81. 1b39
82. 1m83
83. 1r10
84. 1t44
85. Bio1_000406
86. Bio1_000895
87. Bio1_001384
88. Adenosine Triphosphate [mi]
89. Act02649
90. Amy22292
91. Hy-b2176
92. Zinc4261765
93. Tox21_112044
94. Adenosine Triphosphate [inci]
95. Bdbm50366480
96. S5260
97. Adenosine Triphosphate [vandf]
98. Adenosine Triphosphate [mart.]
99. Akos022179934
100. Adenosine Triphosphate [who-dd]
101. Cs-7674
102. Db00171
103. Cas-56-65-5
104. Adenosine 5'-triphosphate; Triphosphaden
105. [[(2r,3s,4r,5r)-5-(6-aminopurin-9-yl)-3,4-dihydroxy-tetrahydrofuran-2-yl]methoxy-hydroxy-phosphoryl] Phosphono Hydrogen Phosphate
106. 5'-(tetrahydrogen Triphosphate) Adenosine
107. Ac-32151
108. As-19204
109. Db-022415
110. C00002
111. D08646
112. D70159
113. Q80863
114. 065a467
115. A831137
116. W-105506
117. A9788c43-4bc5-46e2-8560-41c5fa7d3aa3
118. 9h-purin-6-amine, 9-[5-o-[hydroxy[[hydroxy(phosphonooxy)phosphinyl]oxy]phosphinyl]
119. [(2r,3s,4r,5r)-5-(6-aminopurin-9-yl)-3,4-dihydroxyoxolan-2-yl]methyl (hydroxy-phosphonooxyphosphoryl) Hydrogen Phosphate
120. [(2r,3s,4r,5r)-5-(6-aminopurin-9-yl)-3,4-dihydroxyoxolan-2-yl]methyl (hydroxy-phosphonooxyphosphoryl) Hydrogen Phosphate;((2r,3s,4r,5r)-5-(6-amino-9h-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl Tetrahydrogen Triphosphate
121. [[[(2r,3s,4r,5r)-5-(6-aminopurin-9-yl)-3,4-dihydroxy-oxolan-2-yl]methoxy-hydroxy-phosphoryl]oxy-hydroxy-phosphoryl]oxyphosphonic Acid
122. 9h-purin-6-amine, 9-[5-o-[hydroxy[[hydroxy(phosphonooxy)phosphinyl]oxy]phosphinyl]-.beta.-d-ribofuranosyl]-
| Molecular Weight | 507.18 g/mol |
|---|---|
| Molecular Formula | C10H16N5O13P3 |
| XLogP3 | -5.7 |
| Hydrogen Bond Donor Count | 7 |
| Hydrogen Bond Acceptor Count | 17 |
| Rotatable Bond Count | 8 |
| Exact Mass | 506.99574658 g/mol |
| Monoisotopic Mass | 506.99574658 g/mol |
| Topological Polar Surface Area | 279 Ų |
| Heavy Atom Count | 31 |
| Formal Charge | 0 |
| Complexity | 800 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For nutritional supplementation, also for treating dietary shortage or imbalance
Adenosine triphosphate (ATP) is the nucleotide known in biochemistry as the "molecular currency" of intracellular energy transfer; that is, ATP is able to store and transport chemical energy within cells. ATP also plays an important role in the synthesis of nucleic acids. The total quantity of ATP in the human body is about 0.1 mole. The energy used by human cells requires the hydrolysis of 200 to 300 moles of ATP daily. This means that each ATP molecule is recycled 2000 to 3000 times during a single day. ATP cannot be stored, hence its consumption must closely follow its synthesis.
ATP is able to store and transport chemical energy within cells. ATP also plays an important role in the synthesis of nucleic acids. ATP can be produced by various cellular processes, most typically in mitochondria by oxidative phosphorylation under the catalytic influence of ATP synthase. The total quantity of ATP in the human body is about 0.1 mole. The energy used by human cells requires the hydrolysis of 200 to 300 moles of ATP daily. This means that each ATP molecule is recycled 2000 to 3000 times during a single day. ATP cannot be stored, hence its consumption must closely follow its synthesis.
Market Place

ABOUT THIS PAGE
53
PharmaCompass offers a list of Adenosine Triphosphate API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Adenosine Triphosphate manufacturer or Adenosine Triphosphate supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Adenosine Triphosphate manufacturer or Adenosine Triphosphate supplier.
PharmaCompass also assists you with knowing the Adenosine Triphosphate API Price utilized in the formulation of products. Adenosine Triphosphate API Price is not always fixed or binding as the Adenosine Triphosphate Price is obtained through a variety of data sources. The Adenosine Triphosphate Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Adenosine Triphosphate manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Adenosine Triphosphate, including repackagers and relabelers. The FDA regulates Adenosine Triphosphate manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Adenosine Triphosphate API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Adenosine Triphosphate supplier is an individual or a company that provides Adenosine Triphosphate active pharmaceutical ingredient (API) or Adenosine Triphosphate finished formulations upon request. The Adenosine Triphosphate suppliers may include Adenosine Triphosphate API manufacturers, exporters, distributors and traders.
click here to find a list of Adenosine Triphosphate suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Adenosine Triphosphate DMF (Drug Master File) is a document detailing the whole manufacturing process of Adenosine Triphosphate active pharmaceutical ingredient (API) in detail. Different forms of Adenosine Triphosphate DMFs exist exist since differing nations have different regulations, such as Adenosine Triphosphate USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Adenosine Triphosphate DMF submitted to regulatory agencies in the US is known as a USDMF. Adenosine Triphosphate USDMF includes data on Adenosine Triphosphate's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Adenosine Triphosphate USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Adenosine Triphosphate suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Adenosine Triphosphate Drug Master File in Japan (Adenosine Triphosphate JDMF) empowers Adenosine Triphosphate API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Adenosine Triphosphate JDMF during the approval evaluation for pharmaceutical products. At the time of Adenosine Triphosphate JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Adenosine Triphosphate suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Adenosine Triphosphate Drug Master File in Korea (Adenosine Triphosphate KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Adenosine Triphosphate. The MFDS reviews the Adenosine Triphosphate KDMF as part of the drug registration process and uses the information provided in the Adenosine Triphosphate KDMF to evaluate the safety and efficacy of the drug.
After submitting a Adenosine Triphosphate KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Adenosine Triphosphate API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Adenosine Triphosphate suppliers with KDMF on PharmaCompass.
Adenosine Triphosphate Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Adenosine Triphosphate GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Adenosine Triphosphate GMP manufacturer or Adenosine Triphosphate GMP API supplier for your needs.
A Adenosine Triphosphate CoA (Certificate of Analysis) is a formal document that attests to Adenosine Triphosphate's compliance with Adenosine Triphosphate specifications and serves as a tool for batch-level quality control.
Adenosine Triphosphate CoA mostly includes findings from lab analyses of a specific batch. For each Adenosine Triphosphate CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Adenosine Triphosphate may be tested according to a variety of international standards, such as European Pharmacopoeia (Adenosine Triphosphate EP), Adenosine Triphosphate JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Adenosine Triphosphate USP).

