



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0

USA (Orange Book)

Europe

Canada

Australia
0

South Africa
0
Uploaded Dossiers
U.S. Medicaid
0
Annual Reports
0
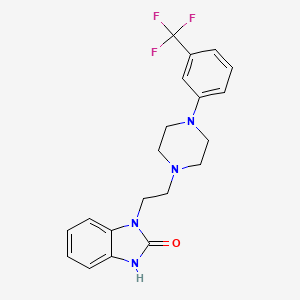

1. 1-(2-(4-(3-trifluoromethylphenyl)piperazin-1-yl)ethyl)benzimidazol(1h)-2-one
2. Addyi
3. Bimt 17
4. Bimt-17
1. 167933-07-5
2. Bimt-17
3. Bimt 17
4. Addyi
5. Ectris
6. Girosa
7. Bimt 17 Bs
8. Bimt-17-bs
9. Filbanserin.
10. 1-(2-(4-(3-(trifluoromethyl)phenyl)piperazin-1-yl)ethyl)-1h-benzo[d]imidazol-2(3h)-one
11. 1,3-dihydro-1-(2-(4-(3-(trifluoromethyl)phenyl)-1-piperazinyl)ethyl)-2h-benzimidazol-2-one
12. 37jk4str6z
13. Female Viagra [street Name]
14. 3-[2-[4-[3-(trifluoromethyl)phenyl]piperazin-1-yl]ethyl]-1h-benzimidazol-2-one
15. 2h-benzimidazol-2-one, 1,3-dihydro-1-[2-[4-[3-(trifluoromethyl)phenyl]-1-piperazinyl]ethyl]-
16. 1-(2-(4-(3-trifluoromethylphenyl)piperazin-1-yl)ethyl)benzimidazol(1h)-2-one
17. Female Viagra (street Name)
18. Flibanserin [usan]
19. 1,3-dihydro-1-[2-[4-[3-(trifluoromethyl)phenyl]-1-piperazinyl]ethyl]-2h-benzimidazol-2-one
20. Flibanserin [usan:inn]
21. Unii-37jk4str6z
22. Addyi (tn)
23. Flibanserin [mi]
24. Flibanserin [inn]
25. Flibanserin (usan/inn)
26. Bimt 17bs
27. Bimt-17bs
28. 1-(2-(4-alpha,alpha,alpha-trifluoro-m-tolyl)-1-piperazinyl)ethyl)-2-benzimidazolinone
29. Flibanserin [mart.]
30. Flibanserin [who-dd]
31. Girosa (proposed Trade Name)
32. Schembl247579
33. Chembl231068
34. Gtpl8182
35. Chebi:90865
36. Hsdb 8278
37. Flibanserin [orange Book]
38. Hms3885k21
39. Bcp02131
40. Ex-a2807
41. Hy-a0095
42. Bdbm50476735
43. Mfcd00918402
44. S3716
45. Zinc52716421
46. Akos005146139
47. Flibanserin; Bimt-17; Bimt-17bs
48. Am84577
49. Ccg-268528
50. Cs-4671
51. Db04908
52. 1-(2-(4-(alpha,alpha,alpha-trifluoro-m-tolyl)-1-piperazinyl)ethyl)-2-benzimidazolinone
53. 2h-benzimidazol-2-one,1,3-dihydro-1-[2-[4-[3-(trifluoromethyl)phenyl]-1-piperazinyl]ethyl]-
54. Ncgc00386567-01
55. Ncgc00386567-02
56. Ncgc00386567-03
57. F1239
58. Ft-0659246
59. D02577
60. 933f075
61. A810922
62. L001463
63. Q415996
64. 1-(2-(4-alpha,alpha,alpha-trifluoro-m-tolyl)-1-(piperazinyl)ethyl)-2-benzimidazolinone
65. 1-[2-[4-(3-trifluoromethylphenyl)piperazin-1-yl]ethyl]benzimidazol-[1h]-2-one
66. 3-[2-[4-[3-(trifluoromethyl)phenyl]-1-piperazinyl]ethyl]-1h-benzimidazol-2-one
67. 1-(2-(4-(.alpha.,.alpha.,.alpha.-trifluoro-m-tolyl)-1-piperazinyl)ethyl)-2-benzimidazolinone
68. 1-(2-{4-[3-(trifluoromethyl)phenyl]piperazin-1-yl}ethyl)-1,3-dihydro-2h-benzimidazol-2-one
| Molecular Weight | 390.4 g/mol |
|---|---|
| Molecular Formula | C20H21F3N4O |
| XLogP3 | 3.3 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 4 |
| Exact Mass | 390.16674579 g/mol |
| Monoisotopic Mass | 390.16674579 g/mol |
| Topological Polar Surface Area | 38.8 Ų |
| Heavy Atom Count | 28 |
| Formal Charge | 0 |
| Complexity | 550 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Flibanserin is included in the database.
NIH/NLM; ClinicalTrials.Gov. Available from, as of September 30, 2015: https://clinicaltrials.gov/search/intervention=FLIBANSERIN%20OR%20BIMT-17
Addyi is indicated for the treatment of premenopausal women with acquired, generalized hypoactive sexual desire disorder (HSDD), as characterized by low sexual desire that causes marked distress or interpersonal difficulty and is NOT due to: A co-existing medical or psychiatric condition, problems within the relationship, or the effects of a medication or other drug substance. Acquired HSDD refers to HSDD that develops in a patient who previously had no problems with sexual desire. Generalized HSDD refers to HSDD that occurs regardless of the type of stimulation, situation or partner. /Included in US product label/
NIH; DailyMed. Current Medication Information for Addyi (Flibanserin) Tablet, Film Coated (Updated: August 2015). Available from, as of November 20, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3819daf3-e935-2c53-c527-e1d57922f394
EXPL THER A central problem in the treatment of Parkinson's disease (PD) is the development of motor disturbances like L-DOPA-induced dyskinesia (LID) after long-term treatment. Preclinical and clinical studies demonstrated that serotonin 5-HT(1A) receptor agonists attenuate this disabling motor side effect. The aim of this study was to investigate the ability of flibanserin compared to buspirone to attenuate L-DOPA-sensitized contraversive circling in hemiparkinsonian rats, which is an animal model of LID. Both drugs have a preferential affinity for the serotonin 5-HT(1A) receptors. Buspirone was in comparison because it was expected to have an effect in this model. Unilaterally 6-hydroxydopamine lesioned rats were treated twice daily intraperitoneally (ip) with L-DOPA methylester (12.5 mg/kg) and benserazide (3.25 mg/kg) for 21 days (on days 1, 3, 5, 8, 11, 14, 17 and 21). On day 24, L-DOPA-sensitized rats were treated ip 5 min prior to administration of L-DOPA methyl ester and benserazide with either saline (controls), 2.5, 5 and 10 mg/kg buspirone or flibanserin. Acute administration of both flibanserin and buspirone, dose dependently, attenuated the increased contraversive circling. An almost complete inhibition of the turning response was observed at 5 mg/kg buspirone and 10 mg/kg flibanserin. The current preclinical findings further implicate the 5-HT(1A) receptor as a promising therapeutic target for the reduction of LID and predict a potential efficacy of flibanserin in the treatment of LID in PD.
PMID:21274579 Gerlach M et al; J Neural Transm 118(12): 1727-32 (2011).
/BOX WARNING/ The use of Addyi in patients with hepatic impairment increases flibanserin concentrations, which can cause severe hypotension and syncope. Therefore, Addyi is contraindicated in patients with hepatic impairment. Inhibitors are contraindicated in patients taking Addyi.
NIH; DailyMed. Current Medication Information for Addyi (Flibanserin) Tablet, Film Coated (Updated: August 2015). Available from, as of November 20, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3819daf3-e935-2c53-c527-e1d57922f394
/BOX WARNING/ The concomitant use of Addyi and moderate or strong CYP3A4 inhibitors increase s flibanserin concentrations, which can cause severe hypotension and syncope. Therefore, the use of moderate or strong CYP3A4 inhibitors is contraindicated in patients taking Addyi.
NIH; DailyMed. Current Medication Information for Addyi (Flibanserin) Tablet, Film Coated (Updated: August 2015). Available from, as of November 20, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3819daf3-e935-2c53-c527-e1d57922f394
/BOX WARNING/ The use of Addyi and alcohol increases the risk of severe hypotension and syncope. Therefore, alcohol use is contraindicated in patients taking Addyi. Before prescribing Addyi, assess the likelihood of the patient abstaining from alcohol, taking into account the patient's current and past drinking behavior, and other pertinent social and medical history. Counsel patients who are prescribed Addyi about the importance of abstaining from alcohol use. Because of the increased risk of hypotension and syncope due to an interaction with alcohol, Addyi is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the Addyi REMS Program.
NIH; DailyMed. Current Medication Information for Addyi (Flibanserin) Tablet, Film Coated (Updated: August 2015). Available from, as of November 20, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3819daf3-e935-2c53-c527-e1d57922f394
Addyi can cause CNS depression (e.g., somnolence, sedation). In five 24-week, randomized, placebo controlled, double-blind trials of premenopausal women with hypoactive sexual desire disorder (HSDD), the incidence of somnolence, sedation or fatigue was 21% and 8% in patients treated with 100 mg Addyi once daily at bedtime and placebo, respectively. The risk of CNS depression is increased if Addyi is taken during waking hours, or if Addyi is taken with alcohol or other CNS depressants, or with medications that increase flibanserin concentrations, such as CYP3A4 inhibitors.
NIH; DailyMed. Current Medication Information for Addyi (Flibanserin) Tablet, Film Coated (Updated: August 2015). Available from, as of November 20, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3819daf3-e935-2c53-c527-e1d57922f394
For more Drug Warnings (Complete) data for Flibanserin (9 total), please visit the HSDB record page.
For the treatment of hypoactive sexual desire disorder (HSDD) in premenopausal women.
FDA Label
Hypoactive Sexual Desire Disorder in Women
G - Genito urinary system and sex hormones
G02 - Other gynecologicals
G02C - Other gynecologicals
G02CX - Other gynecologicals
G02CX02 - Flibanserin
Absorption
Flibanserin has an absolute oral availability of 33%.
Route of Elimination
Elimination via feces (51%) and urine (44%) following a single oral 50 mg dose of flibanserin solution.
Approximately 98% of the drug is bound to human serum proteins, mainly to albumin.
NIH; DailyMed. Current Medication Information for Addyi (Flibanserin) Tablet, Film Coated (Updated: August 2015). Available from, as of November 20, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3819daf3-e935-2c53-c527-e1d57922f394
Food increased the extent of absorption and slowed the rate of absorption of a 50 mg dose of flibanserin (one half the recommended dosage). Low-, moderate-, and high-fat meals increased flibanserin AUC0-inf by 1.18-, 1.43-, and 1.56-fold; increased Cmax by 1.02-, 1.13-, and 1.15-fold; and prolonged median Tmax to 1.5, 0.9, 1.8 hours from 0.8 hours under fasted conditions, respectively.
NIH; DailyMed. Current Medication Information for Addyi (Flibanserin) Tablet, Film Coated (Updated: August 2015). Available from, as of November 20, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3819daf3-e935-2c53-c527-e1d57922f394
Following oral ministration of a single 100 mg dose of flibanserin in healthy premenopausal women (N=8), mean (SD) Cmax was 419 (206) ng/mL and mean (SD) AUC0-inf was 1543 (511) ng*hr/mL. Median (range) time to reach Cmax was 0.75 (0.75 to 4.0) hours. Absolute bioavailability of flibanserin following oral dosing is 33%.
NIH; DailyMed. Current Medication Information for Addyi (Flibanserin) Tablet, Film Coated (Updated: August 2015). Available from, as of November 20, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3819daf3-e935-2c53-c527-e1d57922f394
/MILK/ Flibanserin is excreted in rat milk. It is unknown whether flibanserin is present in human milk, ... .
NIH; DailyMed. Current Medication Information for Addyi (Flibanserin) Tablet, Film Coated (Updated: August 2015). Available from, as of November 20, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3819daf3-e935-2c53-c527-e1d57922f394
Metabolism is primarily via CYP3A4, slightly CYP2C19. Minimal involvement of CYP1A2, CYP2B6, CYP2C8, CYP2C9 or CYP2D6. At least 35 metabolites of flibanserin are produced, 2 of which reach plasma concentrations as high as parent drug, however they are pharmacologically inactive.
Flibanserin is primarily metabolized by CYP3A4 and, to a lesser extent, by CYP2C19. Based on in vitro and/or in vivo data, CYP1A2, CYP2B6, CYP2C8, CYP2C9, and CYP2D6 contribute minimally to the metabolism of flibanserin. After a single oral solution dose of 50 mg 14C-radiolabeled flibanserin, 44% of the total 14C-flibanserin related radioactivity was recovered in urine, and 51% was recovered in feces. Flibanserin is extensively metabolized to at least 35 metabolites, most of them occurring in low concentrations in plasma. Two metabolites could be characterized that showed plasma concentrations similar to that achieved with flibanserin: 6,21-dihydroxy-flibanserin-6,21-disulfate and 6-hydroxy-flibanserin-6-sulfate. These two metabolites are inactive.
NIH; DailyMed. Current Medication Information for Addyi (Flibanserin) Tablet, Film Coated (Updated: August 2015). Available from, as of November 20, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3819daf3-e935-2c53-c527-e1d57922f394
11 hours
Flibanserin has a mean terminal half-life of approximately 11 hours.
NIH; DailyMed. Current Medication Information for Addyi (Flibanserin) Tablet, Film Coated (Updated: August 2015). Available from, as of November 20, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3819daf3-e935-2c53-c527-e1d57922f394
Flibansetrin has high affinity for serotonin receptors in the brain: it acts as an agonist on 5-HT1A and an antagonist on 5-HT2A. In vivo, flibanserin binds equally to 5-HT1A and 5-HT2A receptors. However, under higher levels of brain 5-HT (i.e., under stress), flibanserin may occupy 5-HT2A receptors in higher proportion than 5-HT(1A) receptors. It may also moderately antagonize D4 (dopamine) receptors and 5-HT2B and 5-HTB2C. Its action on neurotransmitter receptors may contribute to reduction in serotonin levels and increase in dopamine and norepinephrine levels, all of which may play part in reward processing.
Flibanserin has preferential affinity for serotonin 5-HT(1A), dopamine D(4k), and serotonin 5-HT(2A) receptors. In vitro and in microiontophoresis, flibanserin behaves as a 5-HT(1A) agonist, a very weak partial agonist on dopamine D(4) receptors, and a 5-HT(2A) antagonist. In vivo flibanserin binds equally to 5-HT(1A) and 5-HT(2A) receptors. However, under higher levels of brain 5-HT (i.e., under stress), flibanserin may occupy 5-HT(2A) receptors in higher proportion than 5-HT(1A) receptors. The effects of flibanserin on adenylyl cyclase are different from those of buspirone and 8-OH-DPAT, two other purported 5-HT(1A) receptor agonists. Flibanserin reduces neuronal firing rate in cells of the dorsal raphe, hippocampus, and cortex with the CA1 region being the most sensitive in the brain. Flibanserin-induced reduction in firing rate in the cortex seems to be mediated through stimulation of postsynaptic 5-HT(1A) receptors, whereas the reduction of the number of active cells seems to be mediated through dopamine D(4) receptor stimulation. Flibanserin quickly desensitizes somatic 5-HT autoreceptors in the dorsal raphe and enhances tonic activation of postsynaptic 5-HT(1A) receptors in the CA3 region. Flibanserin preferentially reduces synthesis and extracellular levels of 5-HT in the cortex, where it enhances extracellular levels of NE and DA. Flibanserin displays antidepressant-like activity in most animal models sensitive to antidepressants. Such activity, however, seems qualitatively different from that exerted by other antidepressants. Flibanserin seems to act via direct or indirect stimulation of 5-HT(1A), DA, and opioid receptors in those animal models. Flibanserin does not display consistent effects in animal models of anxiety and seems to exert potential antipsychotic effects. Flibanserin may induce some sedation but does not induce observable toxic effects at pharmacologically relevant doses.
PMID:12177684 Borsini F et al; CNS Drug Rev 8(2): 117-42 (2002).
Flibanserin is a novel pharmacologic agent in late-stage clinical testing for hypoactive sexual desire disorder (HSDD) in premenopausal women. A literature review was conducted of all published works on flibanserin and on related studies of serotonin (5-HT)(1A) receptors and 5-HT(2A) receptors, including their actions on monoamines and on sexual function. At clinically relevant doses, flibanserin acts predominantly at 5-HT(1A) receptors as an agonist and secondarily at 5-HT(2A) receptors as an antagonist. Additional binding actions within an order of magnitude of its 5-HT(1A) affinity, which are not likely to be clinically relevant, include weaker antagonist actions at 5-HT(2C) and 5-HT(2B) receptors, and less defined activity at dopamine (DA) D4 receptors. The 5-HT(1A) actions of flibanserin are only seen postsynaptically, which is unlike other agents such as buspirone that act at presynaptic 5-HT(1A) receptors. Furthermore, the postsynaptic actions of chronic flibanserin administration appear to demonstrate a preference for some populations of postsynaptic 5-HT receptors, particularly those that are located on the prefrontal cortex (PFC) pyramidal neurons, which regulate monoamine release in certain selective brain regions. The regional selectivity of flibanserin results in a unique pattern of monoamine modulation. Sustained increases in baseline of DA and norepinephrine (NE) are observed in the PFC, and flibanserin dosing increases DA and NE levels above the basal changes. Conversely, flibanserin induces transient decreases in 5-HT levels in some brain areas such as the PFC, nucleus accumbens, and hypothalamus, but not in other brain areas such as the hippocampus. Therefore, since DA and NE are excitatory and 5-HT is inhibitory to sexual desire and arousal, it is tempting to postulate that the actions of flibanserin on serotonin receptors at the PFC pyramidal neurons, resulting in increased DA and NE yet reduced 5-HT in the PFC, are the mechanistic underpinnings of enhancing sexual desire in HSDD.
PMID:20840530 Stahl SM et al; J Sex Med 8(1):15-27 (2011).
In vitro, flibanserin demonstrated high affinity for the following serotonin (5-hydroxytryptamine or 5-T) receptors: agonist activity at 5-HT1A and antagonist activity at 5-HT2A. Flibanserin also has moderate antagonist activities at the 5-HT2B, 5-HT2C, and dopamine D4 receptors.
NIH; DailyMed. Current Medication Information for Addyi (Flibanserin) Tablet, Film Coated (Updated: August 2015). Available from, as of November 20, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3819daf3-e935-2c53-c527-e1d57922f394






