
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
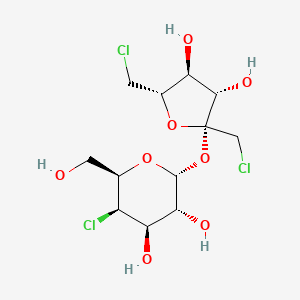

1. Trichlorosucrose
2. 1',4',6'-trichloro-1',4,6'-trideoxygalactosucrose
3. 1,6-dichloro-1,6-dideoxy-beta-d-fructofuranosyl-4-chloro-4-deoxy-alpha-d-galactopyranoside
4. Trichlorogalacto-sucrose
5. Trichlorogalactosucrose
6. Splenda
1. 56038-13-2
2. Trichlorosucrose
3. Splenda
4. Aspasvit
5. Trichlorogalacto-sucrose
6. 96k6uq3zd4
7. Sucrazit
8. Trichlorogalactosucrose
9. Chebi:32159
10. Sansweet Su 100
11. Dtxsid1040245
12. 4,1',6'-trichlorogalactosucrose
13. San Sweet Sa 8020
14. 1,6-dichloro-1,6-dideoxy-beta-d-fructofuranosyl 4-chloro-4-deoxy-alpha-d-galactopyranoside
15. 1,6-dichloro-1,6-dideoxy-beta-d-fructofuranosyl-4-chloro-4-deoxy-alpha-d-galactopyranoside
16. Nsc-759272
17. Ins No.955
18. Dtxcid9020245
19. Ins-955
20. Alpha-d-galactopyranoside, 1,6-dichloro-1,6-dideoxy-beta-d-fructofuranosyl 4-chloro-4-deoxy-
21. 1',4',6'-trichloro-galactosucrose
22. E-955
23. (2r,3r,4r,5r,6r)-2-[(2r,3s,4s,5s)-2,5-bis(chloromethyl)-3,4-dihydroxy-tetrahydrofuran-2-yl]oxy-5-chloro-6-(hydroxymethyl)tetrahydropyran-3,4-diol
24. (2r,3r,4r,5r,6r)-2-((2r,3s,4s,5s)-2,5-bis(chloromethyl)-3,4-dihydroxy-tetrahydrofuran-2-yl)oxy-5-chloro-6-(hydroxymethyl)tetrahydropyran-3,4-diol
25. Refchem:6267
26. Glytoucan:g30984wq
27. 1',4',6'-trichloro-1',4,6'-trideoxygalactosucrose
28. G30984wq
29. 259-952-2
30. 1',4,6'-trichlorogalactosucrose
31. Einecs 259-952-2
32. Mfcd03648615
33. C12h19cl3o8
34. Chembl3185084
35. A-d-galactopyranoside, 1,6-dichloro-1,6-dideoxy-b-d-fructofuranosyl4-chloro-4-deoxy-
36. (2r,3r,4r,5r,6r)-2-(((2r,3s,4s,5s)-2,5-bis(chloromethyl)-3,4-dihydroxytetrahydrofuran-2-yl)oxy)-5-chloro-6-(hydroxymethyl)tetrahydro-2h-pyran-3,4-diol
37. Acucar Light
38. (2r,3r,4r,5r,6r)-2-[(2r,3s,4s,5s)-2,5-bis(chloromethyl)-3,4-dihydroxyoxolan-2-yl]oxy-5-chloro-6-(hydroxymethyl)oxane-3,4-diol
39. .alpha.-d-galactopyranoside, 1,6-dichloro-1,6-dideoxy-.beta.-d-fructofuranosyl 4-chloro-4-deoxy-
40. 4,1',6'-trichloro-4,1',6'-trideoxy-galacto-sucrose
41. E955;trichlorosucrose
42. Unii-96k6uq3zd4
43. Cas-56038-13-2
44. Brn 3654410
45. Sucralose [ban:nf]
46. Ccris 8449
47. Hsdb 7964
48. Sucralose; 1,6-dichloro-1,6-dideoxy-beta-d-fructofuranosyl 4-chloro-4-deoxy-alpha-d-galactopyranoside
49. E955
50. Sucralose [fcc]
51. Sucralose [ii]
52. Sucralose [mi]
53. Sucralose [mart.]
54. Schembl3686
55. Sucralose [usp-rs]
56. Sucralose [who-dd]
57. 1,6-dichloro-1,6-dideoxy-beta-d-fructofuranosyl 4-chloro-4-deoxy-alpha-d-galactose
58. Sucralose, Analytical Standard
59. Orb1308737
60. Sucralose [ep Monograph]
61. Msk3215
62. Baqavosozgmprm-qbmzzyirsa-n
63. Hms2093h16
64. Pharmakon1600-01505953
65. Hy-n0614
66. Sucralose, >=98.0% (hplc)
67. Tox21_113658
68. Tox21_201752
69. Tox21_303425
70. Bdbm50367128
71. Nsc759272
72. S4214
73. Akos015962432
74. Ccg-213995
75. Cs-8130
76. Nsc 759272
77. Os04165
78. Ncgc00249110-01
79. Ncgc00249110-03
80. Ncgc00249110-04
81. Ncgc00257400-01
82. Ncgc00259301-01
83. 1-(1,6-dichloro-1,6-dideoxy-beta-d-fructofuranosyl)-4-chloro-4-deoxy-alpha-d-galactopyranoside
84. Sbi-0206860.p001
85. Sucralose 1000 Microg/ml In Acetonitrile
86. Ns00000320
87. Ab01563242_01
88. Ab01563242_02
89. 038s132
90. Q410209
91. Sr-05000001935
92. Sr-05000001935-1
93. Brd-k58968598-001-02-8
94. Brd-k58968598-001-03-6
95. Sucralose, European Pharmacopoeia (ep) Reference Standard
96. Sucralose, United States Pharmacopeia (usp) Reference Standard
97. Sucralose, Pharmaceutical Secondary Standard; Certified Reference Material
98. 1,6-dichloro-1,6-dideoxy-b-d-fructofuranosyl-4-chloro-4-deoxy-a-d-galactopyranoside
99. 1,6-dichloro-1,6-dideoxy-.beta.-d-fructofuranosyl-4-chloro-4-deoxy-.alpha.-d-galactopyranoside
| Molecular Weight | 397.6 g/mol |
|---|---|
| Molecular Formula | C12H19Cl3O8 |
| XLogP3 | -1.5 |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 5 |
| Exact Mass | Da |
| Monoisotopic Mass | Da |
| Topological Polar Surface Area | 129 |
| Heavy Atom Count | 23 |
| Formal Charge | 0 |
| Complexity | 405 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 9 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Sweetening Agents
Substances that sweeten food, beverages, medications, etc., such as sugar, saccharine or other low-calorie synthetic products. (From Random House Unabridged Dictionary, 2d ed)
After single intravenous doses of (14)C-trichlorogalactosucrose to dogs at a dose level of 2 mg/kg (5.8 uCi/kg) radioactivity was rapidly excreted mainly in the urine. Urinary excretion accounted for means of 29.3%, 63.9% and 74.1% of the dose during 3, 6 and 12 hours after dosing respectively, increasing to 80.9% of the dose after 5 days. Fecal excretion accounted for a mean of 10.4% dose after 24 hours, increasing to 11.9% dose after 5 days. Plasma radioactivity was maximal at 5 minutes after dosing (the first time of sampling, 8.46 ug equivalents/mL). Radioactivity in plasma declined in a multi-exponential fashion; concentrations decreased rapidly to a mean of 0.057 ug equivalents/ml at 12 hours after dosing but thereafter declined more slowly, and were still detectable in all animals at 120 hours after dosing (mean, 0.013 ug equivalents/ml). Consideration of whole-blood and plasma concentrations indicated that radioactivity was cleared more slowly from blood cells than from plasma.
WHO/FAO: Expert Committee on Food Additives. Summary of Toxicological Data of Certain Food Additives Series 24: Trichlorogalactosucrose (1989). Available from, as of July 19, 2011: https://www.inchem.org/pages/jecfa.html
(14)C-trichlorogalactosucrose (1 mg/kg; 100 uCi > 98% pure) was given orally dissolved in water to 8 normal, healthy male volunteers and blood, urine and feces collected for up to 5 days after the dose. The total recovery of (14)C-activity was 92.7% (range 87.8-99.2%) with most of the radioactivity 78.3% (range 69.4-89.6%) in the feces, and the remainder 14.4% (range 8.8-21.7%) in the urine. The plasma concentrations of (14)C-activity reached a peak at about 2 hr after the dose, with levels of (14)C equivalent to approximately 250 ng/mL of trichlorogalactosucrose. The plasma concentrations fell rapidly between 2 and 12 hr followed by a more gradual decrease until 72 hr by which time the levels of radioactivity were near or below the limit of accurate determination. The mean 'effective half-life' calculated on the basis of a mean residence time (MRT) of 18.8 hr gives a value of 13.0 hr.
WHO/FAO: Expert Committee on Food Additives. Summary of Toxicological Data of Certain Food Additives Series 24: Trichlorogalactosucrose (1989). Available from, as of July 19, 2011: https://www.inchem.org/pages/jecfa.html
Three male subjects given a single oral dose (1.11 mg/kg b.w., 0.3 uCi/kg) of trichlorogalactosucrose uniformly labelled with carbon-14 excreted an average of 13.5% of the radioactivity in urine and 82.1% in feces in 5 days. No (14)CO2 was detected in expired air collected during the initial 8 hours after dosing. Maximum levels of radioactivity in the blood occurred within 2-3 hours and in two of the subjects declined with a half-life of approximately 2.5 hours. Chromatographic examination of the 0-3 hours urines indicated the presence of only a single radioactive component.
WHO/FAO: Expert Committee on Food Additives. Summary of Toxicological Data of Certain Food Additives Series 24: Trichlorogalactosucrose (1989). Available from, as of July 19, 2011: https://www.inchem.org/pages/jecfa.html
After single oral doses of (14)C-trichlorogalactosucrose to non-pregnant and pregnant rabbits at a dose level of 10 mg/kg, radioactivity was excreted mainly in the feces. During 24 hours after dosing, a mean of 16.8% of the dose was excreted in the feces of non-pregnant animals, increasing to 31.8% during 48 hours and 54.7% during 120 hours. Excretion of radioactivity in the feces of pregnant rabbits was similar, with means of 27.8%, 43.0% and 65.2% of the dose excreted by this route during 24, 48 and 120 hours after dosing, respectively. Means of 5.3% and 4.2% dose were excreted in the feces of non-pregnant and pregnant rabbits respectively during 96-120 hours after dosing, indicating that excretion of radioactivity was not completed after 5 days, probably because of the coprophagic behavior of rabbits. During 24 hours, means of 8.3% and 8.6% of the dose were excreted in the urine of non-pregnant and pregnant rabbits, respectively. Mean totals of 22.3% (non-pregnant rabbits) and 21.5% (pregnant rabbits) of the dose was gradually excreted in the urine during 5 days after dosing. Radioactivity was still being excreted in the urine of rabbits (up to 2.9% dose) during 96-120 hours after dosing. Mean total recoveries of radioactivity from the urine and feces of non- pregnant and pregnant rabbits after 5 days accounted for 80.3% and 87.0% of the dose respectively. The dose not accounted for was presumably still to be excreted since a total of up to 8.4% of the dose was excreted during 96-120 hours after dosing. There were no notable differences in the absorption and excretion of single oral doses of (14)C-trichlorogalactosucrose between non-pregnant and pregnant rabbits.
WHO/FAO: Expert Committee on Food Additives. Summary of Toxicological Data of Certain Food Additives Series 24: Trichlorogalactosucrose (1989). Available from, as of July 19, 2011: https://www.inchem.org/pages/jecfa.html
For more Absorption, Distribution and Excretion (Complete) data for Sucralose (14 total), please visit the HSDB record page.
Following a single oral dose of (14)C-sucralose (1mg/kg, 100 microCi) to eight male subjects, a mean of 14.5% (range 8.9 to 21.8%) of the radioactivity was excreted in urine and 78.3% (range 69.4 to 89.6%) in the feces, within 5 days. The total recovery of radioactivity averaged 92.8%. Plasma concentrations of radioactivity were maximal at about 2 hours after dosing. The mean residence time (MRT) for sucralose was 18.8 hr, while the effective half-life for the decline of plasma radioactivity was 13 hr. Two volunteers given a higher oral dose (10 mg/kg, 22.7 uCi) excreted a mean of 11.2% (9.6 and 12.7%) of the radioactivity in urine, and 85.5% (84.1 and 86.8%) in feces over 5 days. The total recovery of radioactivity was 96.7%. The radiolabelled material present in feces was essentially unchanged sucralose. Sucralose was the principal component in the urine together with two more polar components which accounted for only 2.6% of the administered dose (range 1.5 to 5.1% of dose); both metabolites possessed characteristics of glucuronide conjugates of sucralose.
PMID:10882816 Roberts A et al; Food Chem Toxicol 38 Suppl 2: S31-41 (2000)
Three male subjects given a single oral dose (1.11 mg/kg b.w., 0.3 uCi/kg) of trichlorogalactosucrose uniformly labelled with carbon-14 excreted an average of 13.5% of the radioactivity in urine and 82.1% in feces in 5 days. ... Maximum levels of radioactivity in the blood occurred within 2-3 hours and in two of the subjects declined with a half-life of approximately 2.5 hours.
WHO/FAO: Expert Committee on Food Additives. Summary of Toxicological Data of Certain Food Additives Series 24: Trichlorogalactosucrose (1989). Available from, as of July 19, 2011: https://www.inchem.org/pages/jecfa.html
Positive allosteric modulators of the human sweet taste receptor ...developed as a new way of reducing dietary sugar intake .../can be used as/ ...valuable tool molecules to study the general mechanism of positive allosteric modulations of T1R taste receptors. Using chimeric receptors, mutagenesis, and molecular modeling, .../the study/ reveal how ...sweet enhancers follow a similar mechanism as the natural umami taste enhancer molecules. Whereas the sweeteners bind to the hinge region and induce the closure of the Venus flytrap domain of T1R2, the enhancers bind close to the opening and further stabilize the closed and active conformation of the receptor.
PMID:20173095 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2842058 Zhang F, et al; Proc Natl Acad Sci USA 107 (10): 4752-4757 (2010)
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
