



API Suppliers

US DMFs Filed

CEP/COS Certifications

JDMFs Filed
0
Other Certificates
Other Suppliers

USA (Orange Book)

Europe

Canada

Australia
0

South Africa
Uploaded Dossiers
U.S. Medicaid
Annual Reports
0
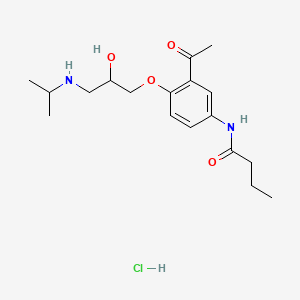

1. Acebutolol
2. Acetobutolol
3. Apo Acebutolol
4. Apo-acebutolol
5. Apoacebutolol
6. M And B 17803 A
7. M And B 17803a
8. M And B-17803 A
9. M And B17803 A
10. Monitan
11. Neptal
12. Novo Acebutolol
13. Novo-acebutolol
14. Novoacebutolol
15. Prent
16. Rhotral
17. Sectral
1. 34381-68-5
2. Acebutolol Hcl
3. Prent
4. Sectral
5. Neptal
6. Acebutolol (hydrochloride)
7. Acetanol
8. N-(3-acetyl-4-(2-hydroxy-3-(isopropylamino)propoxy)phenyl)butyramide Hydrochloride
9. Il-17803a
10. M&b 17803a
11. Nsc-757412
12. Mls000069553
13. N-[3-acetyl-4-[2-hydroxy-3-(propan-2-ylamino)propoxy]phenyl]butanamide;hydrochloride
14. Chebi:2380
15. 3'-acetyl-4'-(2-hydroxy-3-(isopropylamino)propoxy)butyranilide Hydrochloride
16. Dl-1-(2-acetyl-4-butyramidophenoxy)-2-hydroxy-3-isopropylaminopropane Hydrochloride
17. B025y34c54
18. M&b-17803a
19. B 17803a
20. B-17803a
21. Smr000058800
22. (+-)-3'-acetyl-4'-(2-hydroxy-3-(isopropylamino)propoxy)butyranilide Monohydrochloride
23. Dsstox_cid_25461
24. Dsstox_rid_80892
25. N-(3-acetyl-4-{2-hydroxy-3-[(propan-2-yl)amino]propoxy}phenyl)butanamide Hydrochloride
26. N-{3-acetyl-4-[2-hydroxy-3-(propan-2-ylamino)propoxy]phenyl}butanamide Hydrochloride
27. Dsstox_gsid_45461
28. Diasectral
29. Wesfalin
30. N-[3-acetyl-4-[2-hydroxy-3-(propan-2-ylamino)propoxy]phenyl]butanamide Hydrochloride
31. Acebutolol Hydrochloride [jan]
32. Ccris 1102
33. Sr-01000000133
34. Unii-b025y34c54
35. Hsdb 6524
36. Prestwick_512
37. Acebutolol Hydrochloride [usp:jan]
38. Einecs 251-980-3
39. Sectral (tn)
40. Il 17803a
41. M&b 17,803a
42. Opera_id_1259
43. Ncgc00016827-01
44. Cas-34381-68-5
45. Schembl41004
46. Mls001076107
47. Mls002222219
48. Mls002548874
49. Spectrum1500665
50. (+/-)-acebutolol Hydrochloride
51. Chembl1200813
52. Dtxsid5045461
53. Hms1568m19
54. Hms1921a06
55. Pharmakon1600-01500665
56. Bcp28416
57. Tox21_110633
58. Ac-002
59. Acebutolol Hydrochloride [mi]
60. Ccg-39278
61. Hy-17497a
62. Mfcd00078860
63. Nsc757412
64. S4010
65. Acebutolol Hydrochloride (jp17/usp)
66. Akos015895193
67. Tox21_110633_1
68. Ab03018
69. Acebutolol Hydrochloride [hsdb]
70. Ks-5245
71. Nc00669
72. Nsc 757412
73. Acebutolol Hydrochloride [mart.]
74. Acebutolol Hydrochloride [vandf]
75. S11992
76. Acebutolol Hydrochloride [usp-rs]
77. Acebutolol Hydrochloride [who-dd]
78. Ncgc00018215-06
79. Ncgc00094830-01
80. Ncgc00094830-02
81. Ncgc00094830-03
82. Ncgc00094830-04
83. Db-048610
84. Acebutolol Hydrochloride, Analytical Standard
85. Ft-0630582
86. Sw196705-3
87. Acebutolol Hydrochloride [ep Impurity]
88. Acebutolol Hydrochloride [orange Book]
89. Acebutolol Hydrochloride [ep Monograph]
90. Acebutolol Hydrochloride [usp Impurity]
91. Acebutolol Hydrochloride [usp Monograph]
92. C07677
93. D00597
94. 381a685
95. A822205
96. J-019577
97. Sr-01000000133-3
98. Q27105651
99. Acebutolol Hydrochloride 1.0 Mg/ml In Methanol (as Free Base)
100. Acebutolol Hydrochloride, European Pharmacopoeia (ep) Reference Standard
101. Acebutolol Hydrochloride, Pharmaceutical Secondary Standard; Certified Reference Material
102. Acebutolol Hydrochloride, United States Pharmacopeia (usp) Reference Standard
103. Dl-1-(2-acetyl-4-butyramido)-3-(isopropylamino)propan-2-ol Hydrochloride
104. (+/-)-3'-acetyl-4'-(2-hydroxy-3-(isopropylamino)propoxy)butyranilide Monohydrochloride
105. (1)-n-(3-acetyl-4-(2-hydroxy-3-((isopropyl)amino)propoxy)phenyl)butyramide Monohydrochloride
106. Butanamide, N-(3-acetyl-4-(2-hydroxy-3-((1-methylethyl)amino)propoxy)phenyl)-, Monohydrochloride, (+-)-
107. Butanamide, N-(3-acetyl-4-(2-hydroxy-3-((1-methylethyl)amino)propoxy)phenyl)-, Monohydrochloride, (+/-)-
108. Butanamide, N-[3-acetyl-4-[2-hydroxy-3-[(1-methylethyl)amino]propoxy]phenyl]-, Hydrochloride (1:1)
109. Butyranilide, 3'-acetyl-4'-(2-hydroxy-3-(isopropylamino)propoxy)-, Monohydrochloride, (+-)-
110. N-(3-acetyl-4-(2-hydroxy-3-((1-methylethyl)amino)propoxy)phenyl)-, Monohydrochloride, (+/-)-
111. N-[3-acetyl-4-[2-hydroxy-3-(isopropylamino)propoxy]phenyl]butanamide Hydrochloride;acebutolol Hydrochloride
| Molecular Weight | 372.9 g/mol |
|---|---|
| Molecular Formula | C18H29ClN2O4 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 10 |
| Exact Mass | 372.1815851 g/mol |
| Monoisotopic Mass | 372.1815851 g/mol |
| Topological Polar Surface Area | 87.7 Ų |
| Heavy Atom Count | 25 |
| Formal Charge | 0 |
| Complexity | 401 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 2 | |
|---|---|
| Drug Name | Acebutolol hydrochloride |
| Drug Label | Acebutolol HCl is a selective, hydrophilic beta-adrenoreceptor blocking agent with mild intrinsic sympathomimetic activity for use in treating patients with hypertension and ventricular arrhythmias. It is marketed in capsule form for oral administrat... |
| Active Ingredient | Acebutolol hydrochloride |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | eq 200mg base; eq 400mg base |
| Market Status | Prescription |
| Company | Amneal Pharm; Mylan |
| 2 of 2 | |
|---|---|
| Drug Name | Acebutolol hydrochloride |
| Drug Label | Acebutolol HCl is a selective, hydrophilic beta-adrenoreceptor blocking agent with mild intrinsic sympathomimetic activity for use in treating patients with hypertension and ventricular arrhythmias. It is marketed in capsule form for oral administrat... |
| Active Ingredient | Acebutolol hydrochloride |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | eq 200mg base; eq 400mg base |
| Market Status | Prescription |
| Company | Amneal Pharm; Mylan |
Adrenergic beta-Antagonists; Anti-Arrhythmia Agents; Antihypertensive Agents; Sympatholytics
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Acebutolol ... /is/ indicated in the treatment of classic angina pectoris, also referred to as "effort-associated angina". /NOT included in US product labeling/
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 23rd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2003. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 550
Acebutolol /is/ used in the treatment of mitral value prolapse syndrome. /NOT included in US product labeling/
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 23rd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2003. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 551
Acebutolol ... /is/ used for thyrotoxicosis. /NOT included in US product labeling/
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 23rd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2003. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 551
For more Therapeutic Uses (Complete) data for ACEBUTOLOL HYDROCHLORIDE (10 total), please visit the HSDB record page.
Abrupt withdrawal of acebutolol may exacerbate angina symptoms or precipitate myocardial infarction in patients with corornary artery disease. Therefore, patients receiving acebutolol (especially those with ischemic heart disease) should be warned not to interrupt or discontinue therapy without consulting their physician. When acebutolol therapy is discontinued, patients should be monitored carefully and advised to temporarily limit their physical activity. If exacerbation of angina occurs after acebutolol therapy is interrupted, antianginal therapy should be reinstituted promptly, and appropriate measures for the management of unstable angina pectoris should be initiated. Because coronary artery disease is common and may be unrecognized, it may be prudent not to discontinue acebutolol therapy abruptly, even in patients receiving the drug for conditions other than angina.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 1733
Since beta-adrenergic blocking agents may reduce cardiac output and precipitate or aggravate the symptoms of arterial insufficiency in patients with peripheral or mesenteric vascular disease, acebutolol should be used with caution in these patients, and the patients should be observed for evidence of progression of arterial insufficiency.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 1733
... It is recommended that acebutolol be used with caution in patients with diabetes mellitus (especially those with labile diabetes) since the drug also may mask signs and symptoms of hypoglycemia (e.g., tachycardia, palpitation, blood pressure changes, tremor, feelings of anxiety, but not sweating) and may potentiate insulin-induced hypoglycemia.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 1734
Since beta-adrenergic blocking agents may inhibit bronchodilation produced by endogenous catecholamines, the drugs generally should not be used in patients with bronchospastic disease; however, because of its relative beta 1-selective adrenergic blocking activity, acebutolol may be used with caution in patients with bronchospastic disease who do not respond to or cannot tolerate other hypotensive agents.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 1734
For more Drug Warnings (Complete) data for ACEBUTOLOL HYDROCHLORIDE (22 total), please visit the HSDB record page.
Death occurred after an 8.8 g overdose, whereas survival occurred with 7.6 and 9.6 g overdoses.
Ellenhorn, M.J. and D.G. Barceloux. Medical Toxicology - Diagnosis and Treatment of Human Poisoning. New York, NY: Elsevier Science Publishing Co., Inc. 1988., p. 189
Anti-Arrhythmia Agents
Agents used for the treatment or prevention of cardiac arrhythmias. They may affect the polarization-repolarization phase of the action potential, its excitability or refractoriness, or impulse conduction or membrane responsiveness within cardiac fibers. Anti-arrhythmia agents are often classed into four main groups according to their mechanism of action: sodium channel blockade, beta-adrenergic blockade, repolarization prolongation, or calcium channel blockade. (See all compounds classified as Anti-Arrhythmia Agents.)
Adrenergic beta-1 Receptor Antagonists
Drugs that bind to and block the activation of ADRENERGIC BETA-1 RECEPTORS. (See all compounds classified as Adrenergic beta-1 Receptor Antagonists.)
Antihypertensive Agents
Drugs used in the treatment of acute or chronic vascular HYPERTENSION regardless of pharmacological mechanism. Among the antihypertensive agents are DIURETICS; (especially DIURETICS, THIAZIDE); ADRENERGIC BETA-ANTAGONISTS; ADRENERGIC ALPHA-ANTAGONISTS; ANGIOTENSIN-CONVERTING ENZYME INHIBITORS; CALCIUM CHANNEL BLOCKERS; GANGLIONIC BLOCKERS; and VASODILATOR AGENTS. (See all compounds classified as Antihypertensive Agents.)
Sympathomimetics
Drugs that mimic the effects of stimulating postganglionic adrenergic sympathetic nerves. Included here are drugs that directly stimulate adrenergic receptors and drugs that act indirectly by provoking the release of adrenergic transmitters. (See all compounds classified as Sympathomimetics.)
Distribution of acebutolol hydrochloride into body tissue and fluids has not been fully characterized. Following IV administration in rats, acebutolol is distributed extensively into many tissues, including heart, liver, kidneys, lungs, intestines, stomach, and salivary glands, but only minimally into CSF or testes. Following oral administration of acebutolol hydrochloride in healthy individuals, acebutolol and, to a lesser extent, diacetolol, are distributed into saliva and minimally into CSF. Following oral administration of a single 300-mg dose of acebutolol hydrochloride, about 3-9% of the dose is distributed into bile within 24 hours, in approximately equivalent amounts as acebutolol and diacetolol. Peak biliary concentrations of acebutolol are approximately 60-100 times greater than peak plasma concentrations.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 1736
Acebutolol and diacetolol readily cross the placenta and can accumulate in the fetus. In pregnant women receiving acebutolol, the mean acebutolol and diacetolol ratios of umbilical venous to maternal venous plasma concentrations were 0.8 (range: 0.5-1) and 0.6 (range: 0.3-0.8), respectively.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 1736
Following IV administration, acebutolol is rapidly and widely distributed into the extravascular space and the apparent volume of distribution of the drug in healthy adults is approximately 1.6-3 l/kg (range: 1-3.8 l/kg). In healthy individuals, the volume of distribution in the central compartment and at steady state averages 0.16-0.22 and approximately 1.2 l/kg, respectively, following IV administration. The apparent volume distribution may be decreased in geriatric patients.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 1736
In vitro, acebutolol and diacetolol are approximately 11-35 and 6-9% bound, respectively, to plasma proteins at plasma acebutolol concentrations of 20-9,000 ng/ml. Acebutolol is approximately 50% bound to erythrocytes.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 1736
For more Absorption, Distribution and Excretion (Complete) data for ACEBUTOLOL HYDROCHLORIDE (16 total), please visit the HSDB record page.
Acebutolol is rapidly and extensively metabolized in the liver. Acebutolol undergoes extensive hydrolysis of the butyramide group to form the desbutyl primary amine, acetolol, which is almost completely converted via N-acetylation to diacetolol. The extent of metabolism of acebutolol to diacetolol appears to be independent of the genetic acetylator phenotype of the patient. Diacetolol is equipotent to acebutolol and has a similar pharmacologic profile.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 1736
Following single or mutiple oral doses of acebutolol hydrochloride, the elimination half-life of diacetolol reportedly averages 21.5 hours (range: 11-49 hours) or 32 hours (range: 17-54 hours) in patients with creatinine clearances of 6-56 or less than 5 ml/minute, respectively.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 1736
Following a single oral dose in healthy adults, the half-life of acebutolol in the initial distrubution phase (t1/2 alpha) is about 3 hours and the half-life in the terminal phase (t1/2 beta) has been reported to average 11 hours (range: 6-12 hours). The half-lives of the two identified metabolites, diacetolol and acetolol, average 7.5 (range: 7-11 hours) and 3 hours, respectively, following a single oral dose of the drug. The half-life of acebutolol tends to be slightly prolonged following multiple rather than single doses. Following multiple-dose oral administration of acebutolol hyrochloride in healthy individuals (400 mg twice daily for 56 days), the elimination half-life of acebutolol average 13 hours (range: 9-20 hours). The elimination half-lives of acebutolol and diacetolol may be slightly iincreased in geriatric patients.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 1736
The plasma elimination half-lives of acebutolol and diacetolol in neonates born to women receiving the drug during pregnancy ranged from 6-14 and from 24-30 hours, respectively, in the first 24 hours after birth; the half-life of diacetolol decreased to 12-16 hours during the second day. Neonatal urinary excretion of the drug and diacetolol was maximal during the first 24 hours after birth.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 1736
Acebutolol is a beta-selective adrenergic blocking agent and has pharmacologic actions similar to those of other beta-adrenergic blocking agents. At low dosages, acebutolol selectively inhibits response to adrenergic stimuli by competively blocking cardiac beta1-adrenergic receptors, while having little effect on the beta2-adrenergic receptors of bronchial and vascular smooth muscle. At high dosages (eg, greater than 80 mg daily), the selectively of acebutolol for beta-1-adrenergic receptors usually diminishes, and the drug will competively inhibit beta-1- and beta-2-adrenergic receptors. The beta1-selective blocking activity of acebutolol appears to be more pronounced in animals than in humans. In vivo studies in animals and humans indicate that the relative beta-1-adrenergic blocking activity of acebutolol, on a weight basis, is approximately 10-30% that of propranolol, as determined by inhibition of reflex tachycardia in animals or inhibition of exercise or tilt-induced or reflex tachycardia in healthy individuals.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 1735
In addition to inhibiting access of physiologic or synthetic catecholamines to beta-adrenergic receptors, acebutolol exhibits mild intrinsic sympathomimetic activity (partial beta-agonist activity). Acebutolol also has a membrane-stabilizing effect on the heart, which is similar to that of quinidine but occurs only at high plasma concentrations and usually is not apparent at dosages used clinically.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 1735
The pharmacologic effects of acebutolol results from both the unchanged drug and its major metabolite, diacetolol. Diacetolol is equipotent to acebutolol and, in animals, has greater beta-selective adrenergic blocking activity than the parent drug. Diacetolol also has weak intrinsic sympathomimetic activity but does not have substantial membrane-stabilizing activity. Diacetolol may contribute substantially to the observed effects of acebutolol, since plasma concentrations of the metabolite are consistently higher than those of the parent during acebutolol therapy.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 1735






