



API Suppliers

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0

USA (Orange Book)

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
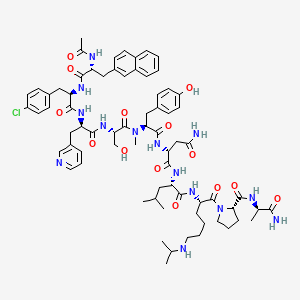

1. Plenaxis
2. Ppi-149
1. Plenaxis
2. 183552-38-7
3. Ppi-149
4. Ppi 149
5. R 3827
6. R3827
7. R-3827
8. Chembl1252
9. Plenaxis Depot
10. Chebi:337298
11. D-alaninamide, N-acetyl-3-(2-naphthalenyl)-d-alanyl-4-chloro-d-phenylalanyl-3-(3-pyridinyl)-d-alanyl-l-seryl-n-methyl-l-tyrosyl-d-asparaginyl-l-leucyl-n6-(1-methylethyl)-l-lysyl-l-prolyl-
12. Abarelix-depot-m
13. Plenaxis (tn)
14. Abarelix (usan/inn)
15. Abarelix [usan:inn]
16. Unii-w486sj5824
17. N-acetyl-3-(2-naphthyl)-d-alanyl-4-chloro-d-phenylalanyl-3-(3-pyridyl)-d-alanyl-l-seryl-n-methyl-l-tyrosyl-d-asparginyl-l-leucyl-n6-isopropyl-l-lysyl-l-prolyl-d-alaninamide
18. Schembl9533
19. Gtpl1188
20. Ppi149
21. Schembl19712245
22. Dtxsid20171443
23. Bdbm50102442
24. Ppi 149r3827
25. R3827;ppi 149
26. Cs-5873
27. Db00106
28. D-alaninamide, N-acetyl-3-(2-naphthalenyl)-d-alanyl-4-chloro-d-phenylalanyl-3-(3-pyridinyl)-d-alanyl-l-seryl-n-methyl-l-tyrosyl-d-asparaginyl-l-leucyl-n6-(1-methylethyl)-l-lysyl-l-prolyl- 10
29. Hy-13534
30. N-acetyl-3-(2-naphthyl)-d-alanyl-4-chloro-d-phenylalanyl-3-(pyridin-3-yl)-d-alanyl-l-seryl-n-methyl-l-tyrosyl-d-asparginyl-l-leucyl-n(6)-isopropyl-l-lysyl-l-prolyl-d-alaninamide
31. A12613
32. D02738
33. 552a387
34. Q305555
35. Ac-d-nal-[d-(pcl)phe]-d-pal-ser-[nalpha-me-tyr]-d-asn-leu-ilys-pro-dala-nh2
36. N-acetyl-3-(2-naphthyl)-d-alanyl-4-chloro-d-phenylalanyl-3-(3-pyridyl)-d-alanyl-l-seryl-n-methyl-l-tyrosyl-d-asparagyl-l-leucyl-n6-isopropyl-l-lysyl-l-prolyl-d-alaninamide
| Molecular Weight | 1416.1 g/mol |
|---|---|
| Molecular Formula | C72H95ClN14O14 |
| XLogP3 | 3.7 |
| Hydrogen Bond Donor Count | 13 |
| Hydrogen Bond Acceptor Count | 16 |
| Rotatable Bond Count | 38 |
| Exact Mass | 1414.6840715 g/mol |
| Monoisotopic Mass | 1414.6840715 g/mol |
| Topological Polar Surface Area | 425 Ų |
| Heavy Atom Count | 101 |
| Formal Charge | 0 |
| Complexity | 2770 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 10 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For palliative treatment of advanced prostate cancer.
FDA Label
Used in the palliative treatment of advanced prostate cancer. Abarelix is a luteinizing hormone agonist that results in suppression of testicular or follicular steroidogenesis.
Hormone Antagonists
Chemical substances which inhibit the function of the endocrine glands, the biosynthesis of their secreted hormones, or the action of hormones upon their specific sites. (See all compounds classified as Hormone Antagonists.)
L - Antineoplastic and immunomodulating agents
L02 - Endocrine therapy
L02B - Hormone antagonists and related agents
L02BX - Other hormone antagonists and related agents
L02BX01 - Abarelix
Absorption
Following IM administration of 100 mg, abarelix is absorbed slowly with a mean peak concentration of 43.4 ng/mL observed approximately 3 days after the injection.
In vitro hepatocyte (rat, monkey, human) studies and in vivo studies in rats and monkeys showed that the major metabolites of abarelix were formed via hydrolysis of peptide bonds. No significant oxidative or conjugated metabolites of abarelix were found either in vitro or in vivo. There is no evidence of cytochrome P-450 involvement in the metabolism of abarelix.
13.2 ± 3.2 days
Abarelix binds to the gonadotropin releasing hormone receptor and acts as a potent inhibitor of gonadotropin secretion.


