
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
Europe
0
Canada
0
Australia
0
South Africa
0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
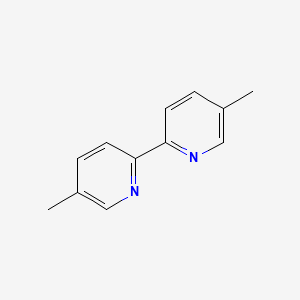

1. 5,5'-dimethyl-2,2'-bipyridyl
2. Dmbp Cpd
1. 1762-34-1
2. 5,5'-dimethyl-2,2'-bipyridine
3. 5,5'-dimethyl-2,2'-dipyridyl
4. 6,6'-bi-3-picoline
5. 5,5'-dimethyl-2,2'-bipyridyl
6. Xeglyze
7. Ha-44
8. 2,2'-bipyridine, 5,5'-dimethyl-
9. 5-methyl-2-(5-methylpyridin-2-yl)pyridine
10. Brn 0123183
11. 6,6'-di-3-picolyl
12. 6,6'-di-3-picoline
13. Ha44
14. 6uo390amfb
15. Chembl2205807
16. 5,5'-dimethyl-2,2'-bipyridinyl
17. Mfcd01740554
18. Abametapir [usan:inn]
19. Unii-6uo390amfb
20. Xeglyze(abametapir)
21. Xeglyze (tn)
22. Abametapir [mi]
23. Abametapir [inn]
24. Abametapir (usan/inn)
25. Abametapir [usan]
26. Abametapir [who-dd]
27. Schembl351152
28. Yssj3184
29. 2,2 -bis-(5-methylpyridyl)
30. Abametapir [orange Book]
31. Dtxsid00170095
32. Chebi:192617
33. Zinc403335
34. Bdbm50401351
35. Lt0042
36. S5752
37. 5,5''-dimethyl-2,2''-bipyridine
38. Akos005257775
39. Cs-w004546
40. Db11932
41. Hy-w004546
42. Sb17220
43. 5,5'-dimethyl-2,2'-dipyridyl, 98%
44. Ds-15219
45. Sy052805
46. Ft-0689891
47. D10687
48. D70523
49. W-108621
50. Q27265547
51. 5,5 Inverted Exclamation Mark -dimethyl-2,2 Inverted Exclamation Mark -bipyridyl
| Molecular Weight | 184.24 g/mol |
|---|---|
| Molecular Formula | C12H12N2 |
| XLogP3 | 2.2 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 1 |
| Exact Mass | 184.100048391 g/mol |
| Monoisotopic Mass | 184.100048391 g/mol |
| Topological Polar Surface Area | 25.8 Ų |
| Heavy Atom Count | 14 |
| Formal Charge | 0 |
| Complexity | 161 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Abametapir is indicated, in the context of an overall lice management program, for the topical treatment of head lice infestation in patients 6 months of age and older.
Abametavir has been shown to inhibit all stages of embryo development in both head and body lice by interfering with enzymes critical to this process. It is relatively unique amongst lice treatments in that it requires only a single application, whereas many current therapies require two applications, due to its exceptional potency and unique mechanism. Its predominant metabolite, abametapir carboxyl, has a prolonged residence time in the body, with an estimated half-life of 71 40 hours or longer in adults - as this metabolite has been shown to inhibit cytochrome P450 enzymes _in vitro_, the use of substrates of CYP3A4, CYP2B6, or CYP1A2 should be avoided for two weeks following the administration of abametapir. Abametapir lotion is formulated with [benzyl alcohol], which has been associated with significant toxicity following unintentional systemic exposure, particularly in neonates and low birth weight infants. Benzyl alcohol-containing formulations should not be administered to patients <6 months of age, and should be administered to pediatric patients cautiously and under direct supervision of an adult to mitigate the risk of unintentional oral ingestion.
P - Antiparasitic products, insecticides and repellents
P03 - Ectoparasiticides, incl. scabicides, insecticides and repellents
P03A - Ectoparasiticides, incl. scabicides
P03AX - Other ectoparasiticides, incl. scabicides
P03AX07 - Abametapir
Absorption
In a pharmacokinetic trial with both adult and pediatric patients, the Cmax and AUC0-8h in the adult group were 41 ng/mL and 121 ng.h/mL and the Cmax and AUC0-8h in the pediatric group were 73 ng/mL and 264 ng.h/mL. In general, systemic exposure to abametapir appears to decrease with increasing age. The median Tmax of abemetapir is 0.57 - 1.54 hours. Following topical administration, benzyl alcohol was found in detectable quantities in the serum of 7 out of 39 pediatric patients. The Cmax of benzyl alcohol in these subjects ranged from 0.52 to 3.57 g/mL. The predominant circulating metabolite of abemetapir (abemtapir carboxylate) is eliminated slowly from the circulation and is therefore found at higher serum concentrations than its parent drug - based on data collected for 72 hours post-administration, the ratios of serum Cmax and AUC0-72h between abametapir and abametapir carboxylate were approximately 30 and 250, respectively.
Route of Elimination
The clearance and excretion of abametapir has not been examined in patients.
Volume of Distribution
Data regarding the volume of distribution of abametapir are not available.
Clearance
The clearance and excretion of abametapir has not been examined in patients.
The biotransformation of abametapir is extensive and primarily mediated by CYP1A2. It is metabolized first to abametapir hydroxyl and then further to abametapir carboxyl - the latter is cleared slowly from the plasma, resulting in higher systemic concentrations than that of the parent drug. _In vitro_ studies suggest that abametapir carboxyl may act as an inhibitor of CYP3A4, CYP2B6, and CYP1A2, particularly at the relatively high and prolonged concentrations observed following topical administration of abametapir.
The elimination half-lives of abametapir and its metabolites have not been well-characterized, but the estimated half-life of abametapir carboxyl is 71 40 hours (or longer) in adults.
There are several metalloproteinases (enzymes requiring metal co-factors to function) involved in the process of louse egg hatching and survival. _In vitro_ studies have demonstrated that metal-chelating agents can inhibit the activity of these proteins, and may therefore be valuable pediculicidal agents. Abametapir is a metalloproteinase inhibitor that targets louse metalloproteinases which are critical to their development and hatching.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Market Place

Patents & EXCLUSIVITIES
ABOUT THIS PAGE
66
PharmaCompass offers a list of Abametapir API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Abametapir manufacturer or Abametapir supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Abametapir manufacturer or Abametapir supplier.
PharmaCompass also assists you with knowing the Abametapir API Price utilized in the formulation of products. Abametapir API Price is not always fixed or binding as the Abametapir Price is obtained through a variety of data sources. The Abametapir Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Abametapir manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Abametapir, including repackagers and relabelers. The FDA regulates Abametapir manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Abametapir API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Abametapir manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Abametapir supplier is an individual or a company that provides Abametapir active pharmaceutical ingredient (API) or Abametapir finished formulations upon request. The Abametapir suppliers may include Abametapir API manufacturers, exporters, distributors and traders.
click here to find a list of Abametapir suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Abametapir DMF (Drug Master File) is a document detailing the whole manufacturing process of Abametapir active pharmaceutical ingredient (API) in detail. Different forms of Abametapir DMFs exist exist since differing nations have different regulations, such as Abametapir USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Abametapir DMF submitted to regulatory agencies in the US is known as a USDMF. Abametapir USDMF includes data on Abametapir's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Abametapir USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Abametapir suppliers with USDMF on PharmaCompass.
Abametapir Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Abametapir GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Abametapir GMP manufacturer or Abametapir GMP API supplier for your needs.
A Abametapir CoA (Certificate of Analysis) is a formal document that attests to Abametapir's compliance with Abametapir specifications and serves as a tool for batch-level quality control.
Abametapir CoA mostly includes findings from lab analyses of a specific batch. For each Abametapir CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Abametapir may be tested according to a variety of international standards, such as European Pharmacopoeia (Abametapir EP), Abametapir JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Abametapir USP).

