
Synopsis
Synopsis
0
JDMF
0
VMF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. 0518, Mk
2. Isentress
3. Mk 0518
4. Mk-0518
5. Mk0518
6. Potassium, Raltegravir
7. Raltegravir Potassium
1. 518048-05-0
2. Mk-0518
3. Isentress
4. Raltegravir (mk-0518)
5. Mk0518
6. Raltegravir (inn)
7. Mk 0518
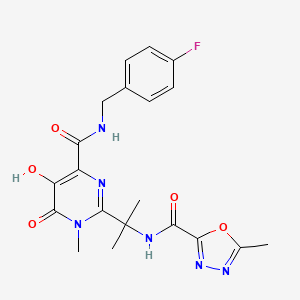
8. N-(2-(4-((4-fluorobenzyl)carbamoyl)-5-hydroxy-1-methyl-6-oxo-1,6-dihydropyrimidin-2-yl)propan-2-yl)-5-methyl-1,3,4-oxadiazole-2-carboxamide
9. 22vkv8053u
10. 518048-05-0 (free)
11. 4-pyrimidinecarboxamide, N-((4-fluorophenyl)methyl)-1,6-dihydro-5-hydroxy-1-methyl-2-(1-methyl-1-(((5-methyl-1,3,4-oxadiazol-2-yl)carbonyl)amino)ethyl)-6-oxo-
12. N-(2-(4-(4-fluorobenzylcarbamoyl)-5-hydroxy-1-methyl-6-oxo-1,6-dihydropyrimidin-2-yl)propan-2-yl)-5-methyl-1,3,4-oxadiazole-2-carboxamide
13. Ncgc00184997-01
14. Raltegravir [inn]
15. Dsstox_cid_28586
16. Dsstox_rid_82857
17. Dsstox_gsid_48660
18. N-[(4-fluorophenyl)methyl]-5-hydroxy-1-methyl-2-{2-[(5-methyl-1,3,4-oxadiazol-2-yl)formamido]propan-2-yl}-6-oxo-1,6-dihydropyrimidine-4-carboxamide
19. Isentress(tm)
20. N-[2-[4-[(4-fluorophenyl)methylcarbamoyl]-5-hydroxy-1-methyl-6-oxopyrimidin-2-yl]propan-2-yl]-5-methyl-1,3,4-oxadiazole-2-carboxamide
21. Cas-518048-05-0
22. Raltegravir [usan:inn]
23. Raltegravirum
24. Unii-22vkv8053u
25. Hsdb 8124
26. N-(4-fluorobenzyl)-5-hydroxy-1-methyl-2-(1-methyl-1-(((5-methyl-1,3,4-oxadiazol-2-yl)carbonyl)amino)ethyl)-6-oxo-1,6-dihydropyrimidine-4-carboxamide
27. N-(4-fluorobenzyl)-5-hydroxy-1-methyl-2-(1-methyl-1-{[(5-methyl-1,3,4-oxadiazol-2-yl)carbonyl]amino}ethyl)-6-oxo-1,6-di Hydropyrimidine-4-carboxamide
28. N-(4-fluorobenzyl)-5-hydroxy-1-methyl-2-(1-methyl-1-{[(5-methyl-1,3,4-oxadiazol-2-yl)carbonyl]amino}ethyl)-6-oxo-1,6-dihydropyrimidine-4-carboxamide
29. Rlt
30. Raltegravir- Bio-x
31. Raltegravir [mi]
32. N-[1-[4-[(4-fluorophenyl)methylcarbamoyl]-5-hydroxy-1-methyl-6-oxo-pyrimidin-2-yl]-1-methyl-ethyl]-5-methyl-1,3,4-oxadiazole-2-carboxamide
33. Raltegravir; Mk-0518
34. K-0518
35. Raltegravir [vandf]
36. Raltegravir - Mk-0518
37. Raltegravir [mart.]
38. Hydropyrimidine-4-carboxamide
39. Schembl51817
40. Raltegravir [who-dd]
41. Mls006011985
42. Schembl996804
43. Raltegravir [ema Epar]
44. Chembl254316
45. Schembl2112870
46. Dtxsid2048660
47. Bdbm25351
48. Chebi:82960
49. Gtpl11571
50. Bcpp000093
51. Hms3655b09
52. Bcp01394
53. Ex-a2147
54. Tox21_113019
55. Mfcd10698872
56. Nsc762522
57. S2005
58. Zinc13831130
59. Akos015902444
60. Akos025149884
61. Akos032960305
62. Tox21_113019_1
63. Zinc114994525
64. Ac-5261
65. Ccg-269170
66. Db06817
67. Nsc-762522
68. Pb13312
69. Sb20935
70. Ncgc00274066-01
71. Ncgc00274066-05
72. As-16992
73. Br164312
74. Hy-10353
75. Smr003601806
76. Ft-0649660
77. Sw220138-1
78. A25486
79. D06676
80. Mk-0518;mk 0518;mk0518
81. Yl}-6-oxo-1,6-dihydropyrimidine-4-carboxamide
82. Ab01566833_01
83. 038r721
84. 048r050
85. A828788
86. Q421552
87. Brd-k05658747-237-01-1
88. [(5-methyl-1,3,4-oxadiazol-2-yl)formamido]propan-2-
89. N-[(4-fluorophenyl)methyl]-5-hydroxy-1-methyl-2-{2-
90. Z1551429745
91. (z)-n-(2-(4-(((4-fluorophenyl)(methyl)amino)(hydroxy)methylene)-1-methyl-5,6-dioxo-1,4,5,6-tetrahydropyrimidin-2-yl)propan-2-yl)-5-methyl-1,3,4-oxadiazole-2-carboxamide
92. Mk-0518;n-[(4-fluorophenyl)methyl]-1,6-dihydro-5-hydroxy-1-methyl-2-[1-methyl-1-[[(5-methyl-1,3,4-oxadiazol-2-yl)carbonyl]amino]ethyl]-6-oxo-4-pyrimidinecarboxamide Potassium Salt
93. N-(2-(4-(4-fluorobenzylcarbamoyl)-5-hydroxy-1-methyl-6-oxo-1, 6-dihydropyrimidin-2-yl)propan-2-yl) -5-methyl-1,3,4-oxadiazole-2- Carboxamide
94. N-(2-(4-(4-fluorobenzylcarbamoyl)-5-hydroxy-1-methyl-6-oxo-1,6- Dihydropyrimidin-2-yl)propan-2-yl)-5-methyl-1,3,4-oxadiazole-2-carboxamide
95. N-(2-(4-(4-fluorobenzylcarbamoyl)-5-oh-1-me-6-oxo-pyrimidin-2-yl)propan-2-yl)-5-me-1,3,4-oxadiazole-2-carboxamide
96. N-(4-fluorobenzyl)-5-hydroxy-1-methyl-2-(1-methyl-1-{[(5-methyl-1,3,4-oxadiazol-2-yl)carbonyl]amino}ethyl)-6-oxo-1,6-di
97. N-(4-fluorobenzyl)-5-hydroxy-1-methyl-2-(2-{[(5-methyl-1,3,4-oxadiazol-2-yl)carbonyl]amino}propan-2-yl)-6-oxo-1,6-dihydropyrimidine-4-carboxamide
98. N-[2-(4-{[(4-fluorophenyl)methyl]carbamoyl}-5-hydroxy-1-methyl-6-oxo-1,6-dihydropyrimidin-2-yl)propan-2-yl]-5-methyl-1,3,4-oxadiazole-2-carboxamide
| Molecular Weight | 444.4 g/mol |
|---|---|
| Molecular Formula | C20H21FN6O5 |
| XLogP3 | 1.1 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 6 |
| Exact Mass | 444.15574595 g/mol |
| Monoisotopic Mass | 444.15574595 g/mol |
| Topological Polar Surface Area | 150 Ų |
| Heavy Atom Count | 32 |
| Formal Charge | 0 |
| Complexity | 836 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Isentress |
| PubMed Health | Raltegravir (By mouth) |
| Drug Classes | Antiretroviral Agent |
| Drug Label | ISENTRESS contains raltegravir potassium, a human immunodeficiency virus integrase strand transfer inhibitor. The chemical name for raltegravir potassium is N-[(4-Fluorophenyl)methyl]-1,6-dihydro-5-hydroxy-1-methyl-2-[1-methyl-1-[[(5-methyl-1,3,4-oxa... |
| Active Ingredient | Raltegravir potassium |
| Dosage Form | Tablet; Powder; Tablet, chewable |
| Route | Oral |
| Strength | eq 100mg base; eq 400mg base; eq 100mg base/packet; eq 25mg base |
| Market Status | Prescription |
| Company | Merck Sharp Dohme |
| 2 of 4 | |
|---|---|
| Drug Name | Raltegravir |
| PubMed Health | Raltegravir (By mouth) |
| Drug Classes | Antiretroviral Agent |
| Drug Label | ISENTRESS contains raltegravir potassium, a human immunodeficiency virus integrase strand transfer inhibitor. The chemical name for raltegravir potassium is N-[(4-Fluorophenyl)methyl]-1,6-dihydro-5-hydroxy-1-methyl-2-[1-methyl-1-[[(5-methyl-1,3,4-oxa... |
| Active Ingredient | Raltegravir |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 400mg |
| Market Status | Tentative Approval |
| Company | Hetero Labs Ltd Iii |
| 3 of 4 | |
|---|---|
| Drug Name | Isentress |
| PubMed Health | Raltegravir (By mouth) |
| Drug Classes | Antiretroviral Agent |
| Drug Label | ISENTRESS contains raltegravir potassium, a human immunodeficiency virus integrase strand transfer inhibitor. The chemical name for raltegravir potassium is N-[(4-Fluorophenyl)methyl]-1,6-dihydro-5-hydroxy-1-methyl-2-[1-methyl-1-[[(5-methyl-1,3,4-oxa... |
| Active Ingredient | Raltegravir potassium |
| Dosage Form | Tablet; Powder; Tablet, chewable |
| Route | Oral |
| Strength | eq 100mg base; eq 400mg base; eq 100mg base/packet; eq 25mg base |
| Market Status | Prescription |
| Company | Merck Sharp Dohme |
| 4 of 4 | |
|---|---|
| Drug Name | Raltegravir |
| PubMed Health | Raltegravir (By mouth) |
| Drug Classes | Antiretroviral Agent |
| Drug Label | ISENTRESS contains raltegravir potassium, a human immunodeficiency virus integrase strand transfer inhibitor. The chemical name for raltegravir potassium is N-[(4-Fluorophenyl)methyl]-1,6-dihydro-5-hydroxy-1-methyl-2-[1-methyl-1-[[(5-methyl-1,3,4-oxa... |
| Active Ingredient | Raltegravir |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 400mg |
| Market Status | Tentative Approval |
| Company | Hetero Labs Ltd Iii |
Pyrrolidinones
National Library of Medicine's Medical Subject Headings online file (MeSH, 2009)
ISENTRESS (raltegravir) is indicated in combination with other antiretroviral agents for the treatment of human immunodeficiency virus (HIV-1) infection. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for ISENTRESS (raltegravir) tablet, film coated; tablet, chewable (Janurary 2013). Available from, as of March 14, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=89a5ec53-d956-4329-8004-0f40f51c88a3
ISENTRESS (raltegravir) is indicated in combination with other antiretroviral agents for the treatment of HIV-1 infection in children and adolescents 2 years of age and older and weighing at least 10 kg. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for ISENTRESS (raltegravir) tablet, film coated; tablet, chewable (Janurary 2013). Available from, as of March 14, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=89a5ec53-d956-4329-8004-0f40f51c88a3
/EXPERIMENTAL THER/ /Investigators/ describe five patients with HIV-2 infection (four antiretroviral-experienced and one antiretroviral-naive) treated with a regimen containing raltegravir. All responded to treatment as demonstrated by viral load and CD4(+) T-cell count monitoring. /This/ series confirms the clinical effectiveness of raltegravir in HIV-2-infected patients when given with other antiretrovirals to which the virus is susceptible.
PMID:22892365 Peterson K et al; Antivir Ther.17 (6):1097-100 (2012)
/EXPERIMENTALTHER VET/ Feline leukemia virus (FeLV) is a gammaretrovirus that is a significant cause of neoplastic-related disorders affecting cats worldwide. Treatment options for FeLV are limited, associated with serious side effects, and can be cost-prohibitive. The development of drugs used to treat a related retrovirus, human immunodeficiency virus type 1 (HIV-1), has been rapid, leading to the approval of five drug classes. Although structural differences affect the susceptibility of gammaretroviruses to anti-HIV drugs, the similarities in mechanism of replication suggest that some anti-HIV-1 drugs may also inhibit FeLV. This study demonstrates the anti-FeLV activity of four drugs approved by the US FDA (Food and Drug Administration) at non-toxic concentrations. Of these, tenofovir and raltegravir are anti-HIV-1 drugs, while decitabine and gemcitabine are approved to treat myelodysplastic syndromes and pancreatic cancer, respectively, but also have anti-HIV-1 activity in cell culture. Our results indicate that these drugs may be useful for FeLV treatment and should be investigated for mechanism of action and suitability for veterinary use.
PMID:22258856 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3542715 Greggs WM 3rd et al; J Gen Virol. 93 (Pt 4): 900-5 (2012)
Severe, potentially life-threatening skin reactions have been reported, including some fatalities. Stevens-Johnson syndrome, toxic epidermal necrolysis, and hypersensitivity reactions characterized by rash, constitutional findings, and organ dysfunction (including hepatic failure) have occurred.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012
Raltegravir and any other suspect agents should be discontinued immediately if signs or symptoms of severe skin or hypersensitivity reactions occur, including (but not limited to) severe rash or rash accompanied by fever, general malaise, fatigue, muscle or joint aches, blisters, oral lesions, conjunctivitis, facial edema, hepatitis, eosinophilia, or angioedema. Clinical status, including liver aminotransferases, should be monitored and appropriate therapy initiated. A life-threatening reaction could occur if there is a delay in discontinuing raltegravir or other suspect agents after the onset of severe rash.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012
During initial treatment, patients who respond to antiretroviral therapy may develop an inflammatory response to indolent or residual opportunistic infections (e.g., Mycobacterium avium, M. tuberculosis, cytomegalovirus (CMV), Pneumocystis jiroveci (formerly P. carinii), varicella-zoster virus (VZV)); this may necessitate further evaluation and treatment. Autoimmune disorders (e.g., Graves' disease, polymyositis, Guillain-Barre syndrome) have been reported to occur in the setting of immune reconstitution; the time to onset is more variable and can occur many months after initiation of antiretroviral therapy.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 691
Individuals with phenylketonuria (i.e., homozygous genetic deficiency of phenylalanine hydroxylase) and other individuals who must restrict their intake of phenylalanine should be advised that raltegravir chewable tablets contain aspartame (NutraSweet), which is metabolized in the GI tract to phenylalanine. Each 25-mg chewable tablet provides approximately 0.05 mg of phenylalanine and each 100-mg chewable tablet provides approximately 0.1 mg of phenylalanine.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012
For more Drug Warnings (Complete) data for Raltegravir (12 total), please visit the HSDB record page.
For the treatment of HIV-1 infection in conjunction with other antiretrovirals.
FDA Label
Isentress is indicated in combination with other anti-retroviral medicinal products for the treatment of human immunodeficiency virus (HIV 1) infection.
Treatment of human immunodeficiency virus (HIV-1) infection
Anti-HIV Agents
Agents used to treat AIDS and/or stop the spread of the HIV infection. These do not include drugs used to treat symptoms or opportunistic infections associated with AIDS. (See all compounds classified as Anti-HIV Agents.)
HIV Integrase Inhibitors
Inhibitors of HIV INTEGRASE, an enzyme required for integration of viral DNA into cellular DNA. (See all compounds classified as HIV Integrase Inhibitors.)
J05AJ01
J05AX08
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
J - Antiinfectives for systemic use
J05 - Antivirals for systemic use
J05A - Direct acting antivirals
J05AJ - Integrase inhibitors
J05AJ01 - Raltegravir
Absorption
Absorbed from the gastrointestinal tract.
Route of Elimination
Feces and urine
Volume of Distribution
Approximately 83% bound to human plasma protein and is minimally distributed into red blood cells (blood-to-plasma partitioning ratio of 0.6).
Clearance
The major mechanism of clearance of raltegravir in humans is glucuronidation mediated by UGT1A1, the renal clearance of unchanged drug is a minor pathway of elimination of raltegravir (9% of total dose).
Raltegravir (film-coated tablet) is absorbed with a Tmax of approximately 3 hours postdose in the fasted state. Raltegravir AUC and Cmax increase dose proportionally over the dose range 100 mg to 1600 mg. Raltegravir C12hr increases dose proportionally over the dose range of 100 to 800 mg and increases slightly less than dose proportionally over the dose range 100 mg to 1600 mg.
US Natl Inst Health; DailyMed. Current Medication Information for ISENTRESS (raltegravir) tablet, film coated; tablet, chewable (Janurary 2013). Available from, as of March 14, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=89a5ec53-d956-4329-8004-0f40f51c88a3
With twice-daily dosing, pharmacokinetic steady state is achieved within approximately the first 2 days of dosing. There is little to no accumulation in AUC and Cmax. The average accumulation ratio for C12hr ranged from approximately 1.2 to 1.6.
US Natl Inst Health; DailyMed. Current Medication Information for ISENTRESS (raltegravir) tablet, film coated; tablet, chewable (Janurary 2013). Available from, as of March 14, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=89a5ec53-d956-4329-8004-0f40f51c88a3
The absolute bioavailability of raltegravir has not been established. Based on a formulation comparison study in healthy adult volunteers, the chewable tablet has higher oral bioavailability compared to the 400 mg film-coated tablet.
US Natl Inst Health; DailyMed. Current Medication Information for ISENTRESS (raltegravir) tablet, film coated; tablet, chewable (Janurary 2013). Available from, as of March 14, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=89a5ec53-d956-4329-8004-0f40f51c88a3
In subjects who received 400 mg twice daily alone, raltegravir drug exposures were characterized by a geometric mean AUC0-12hr of 14.3 uM.hr and C12hr of 142 nM.
US Natl Inst Health; DailyMed. Current Medication Information for ISENTRESS (raltegravir) tablet, film coated; tablet, chewable (Janurary 2013). Available from, as of March 14, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=89a5ec53-d956-4329-8004-0f40f51c88a3
For more Absorption, Distribution and Excretion (Complete) data for Raltegravir (13 total), please visit the HSDB record page.
Hepatic (UGT1A1)
In feces, only raltegravir was present, most of which is likely derived from hydrolysis of raltegravir-glucuronide secreted in bile as observed in preclinical species. Two components, namely raltegravir and raltegravir-glucuronide, were detected in urine and accounted for approximately 9 and 23% of the dose, respectively. The major circulating entity was raltegravir and represented approximately 70% of the total radioactivity; the remaining radioactivity in plasma was accounted for by raltegravir-glucuronide.
US Natl Inst Health; DailyMed. Current Medication Information for ISENTRESS (raltegravir) tablet, film coated; tablet, chewable (Janurary 2013). Available from, as of March 14, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=89a5ec53-d956-4329-8004-0f40f51c88a3
Studies using isoform-selective chemical inhibitors and cDNA-expressed UDP-glucuronosyltransferases (UGT) show that UGT1A1 is the main enzyme responsible for the formation of raltegravir-glucuronide. Thus, the data indicate that the major mechanism of clearance of raltegravir in humans is UGT1A1-mediated glucuronidation.
US Natl Inst Health; DailyMed. Current Medication Information for ISENTRESS (raltegravir) tablet, film coated; tablet, chewable (Janurary 2013). Available from, as of March 14, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=89a5ec53-d956-4329-8004-0f40f51c88a3
9 hours
The apparent terminal half-life of raltegravir is approximately 9 hours, with a shorter alpha-phase half-life (approximetly1 hour) accounting for much of the AUC.
US Natl Inst Health; DailyMed. Current Medication Information for ISENTRESS (raltegravir) tablet, film coated; tablet, chewable (Janurary 2013). Available from, as of March 14, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=89a5ec53-d956-4329-8004-0f40f51c88a3
Raltegravir inhibits HIV integrase to prevent the viral genome being incorporated into the human genome. Raltegravir is primarily metabolized by glucuronidation.
Raltegravir inhibits the catalytic activity of HIV-1 integrase, an HIV-1 encoded enzyme that is required for viral replication. Inhibition of integrase prevents the covalent insertion, or integration, of unintegrated linear HIV-1 DNA into the host cell genome preventing the formation of the HIV-1 provirus. The provirus is required to direct the production of progeny virus, so inhibiting integration prevents propagation of the viral infection. Raltegravir did not significantly inhibit human phosphoryltransferases including DNA polymerases alpha, beta, and gamma.
US Natl Inst Health; DailyMed. Current Medication Information for ISENTRESS (raltegravir) tablet, film coated; tablet, chewable (Janurary 2013). Available from, as of March 14, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=89a5ec53-d956-4329-8004-0f40f51c88a3

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
10
PharmaCompass offers a list of Raltegravir Potassium API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Raltegravir Potassium manufacturer or Raltegravir Potassium supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Raltegravir Potassium manufacturer or Raltegravir Potassium supplier.
PharmaCompass also assists you with knowing the Raltegravir Potassium API Price utilized in the formulation of products. Raltegravir Potassium API Price is not always fixed or binding as the Raltegravir Potassium Price is obtained through a variety of data sources. The Raltegravir Potassium Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A 871038-72-1, Isentress, Raltegravir potassium, MK-518, D07133 manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of 871038-72-1, Isentress, Raltegravir potassium, MK-518, D07133, including repackagers and relabelers. The FDA regulates 871038-72-1, Isentress, Raltegravir potassium, MK-518, D07133 manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. 871038-72-1, Isentress, Raltegravir potassium, MK-518, D07133 API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of 871038-72-1, Isentress, Raltegravir potassium, MK-518, D07133 manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A 871038-72-1, Isentress, Raltegravir potassium, MK-518, D07133 supplier is an individual or a company that provides 871038-72-1, Isentress, Raltegravir potassium, MK-518, D07133 active pharmaceutical ingredient (API) or 871038-72-1, Isentress, Raltegravir potassium, MK-518, D07133 finished formulations upon request. The 871038-72-1, Isentress, Raltegravir potassium, MK-518, D07133 suppliers may include 871038-72-1, Isentress, Raltegravir potassium, MK-518, D07133 API manufacturers, exporters, distributors and traders.
click here to find a list of 871038-72-1, Isentress, Raltegravir potassium, MK-518, D07133 suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A 871038-72-1, Isentress, Raltegravir potassium, MK-518, D07133 DMF (Drug Master File) is a document detailing the whole manufacturing process of 871038-72-1, Isentress, Raltegravir potassium, MK-518, D07133 active pharmaceutical ingredient (API) in detail. Different forms of 871038-72-1, Isentress, Raltegravir potassium, MK-518, D07133 DMFs exist exist since differing nations have different regulations, such as 871038-72-1, Isentress, Raltegravir potassium, MK-518, D07133 USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A 871038-72-1, Isentress, Raltegravir potassium, MK-518, D07133 DMF submitted to regulatory agencies in the US is known as a USDMF. 871038-72-1, Isentress, Raltegravir potassium, MK-518, D07133 USDMF includes data on 871038-72-1, Isentress, Raltegravir potassium, MK-518, D07133's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The 871038-72-1, Isentress, Raltegravir potassium, MK-518, D07133 USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of 871038-72-1, Isentress, Raltegravir potassium, MK-518, D07133 suppliers with USDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a 871038-72-1, Isentress, Raltegravir potassium, MK-518, D07133 Drug Master File in Korea (871038-72-1, Isentress, Raltegravir potassium, MK-518, D07133 KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of 871038-72-1, Isentress, Raltegravir potassium, MK-518, D07133. The MFDS reviews the 871038-72-1, Isentress, Raltegravir potassium, MK-518, D07133 KDMF as part of the drug registration process and uses the information provided in the 871038-72-1, Isentress, Raltegravir potassium, MK-518, D07133 KDMF to evaluate the safety and efficacy of the drug.
After submitting a 871038-72-1, Isentress, Raltegravir potassium, MK-518, D07133 KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their 871038-72-1, Isentress, Raltegravir potassium, MK-518, D07133 API can apply through the Korea Drug Master File (KDMF).
click here to find a list of 871038-72-1, Isentress, Raltegravir potassium, MK-518, D07133 suppliers with KDMF on PharmaCompass.
A 871038-72-1, Isentress, Raltegravir potassium, MK-518, D07133 CEP of the European Pharmacopoeia monograph is often referred to as a 871038-72-1, Isentress, Raltegravir potassium, MK-518, D07133 Certificate of Suitability (COS). The purpose of a 871038-72-1, Isentress, Raltegravir potassium, MK-518, D07133 CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of 871038-72-1, Isentress, Raltegravir potassium, MK-518, D07133 EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of 871038-72-1, Isentress, Raltegravir potassium, MK-518, D07133 to their clients by showing that a 871038-72-1, Isentress, Raltegravir potassium, MK-518, D07133 CEP has been issued for it. The manufacturer submits a 871038-72-1, Isentress, Raltegravir potassium, MK-518, D07133 CEP (COS) as part of the market authorization procedure, and it takes on the role of a 871038-72-1, Isentress, Raltegravir potassium, MK-518, D07133 CEP holder for the record. Additionally, the data presented in the 871038-72-1, Isentress, Raltegravir potassium, MK-518, D07133 CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the 871038-72-1, Isentress, Raltegravir potassium, MK-518, D07133 DMF.
A 871038-72-1, Isentress, Raltegravir potassium, MK-518, D07133 CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. 871038-72-1, Isentress, Raltegravir potassium, MK-518, D07133 CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of 871038-72-1, Isentress, Raltegravir potassium, MK-518, D07133 suppliers with CEP (COS) on PharmaCompass.
A 871038-72-1, Isentress, Raltegravir potassium, MK-518, D07133 written confirmation (871038-72-1, Isentress, Raltegravir potassium, MK-518, D07133 WC) is an official document issued by a regulatory agency to a 871038-72-1, Isentress, Raltegravir potassium, MK-518, D07133 manufacturer, verifying that the manufacturing facility of a 871038-72-1, Isentress, Raltegravir potassium, MK-518, D07133 active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting 871038-72-1, Isentress, Raltegravir potassium, MK-518, D07133 APIs or 871038-72-1, Isentress, Raltegravir potassium, MK-518, D07133 finished pharmaceutical products to another nation, regulatory agencies frequently require a 871038-72-1, Isentress, Raltegravir potassium, MK-518, D07133 WC (written confirmation) as part of the regulatory process.
click here to find a list of 871038-72-1, Isentress, Raltegravir potassium, MK-518, D07133 suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing 871038-72-1, Isentress, Raltegravir potassium, MK-518, D07133 as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for 871038-72-1, Isentress, Raltegravir potassium, MK-518, D07133 API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture 871038-72-1, Isentress, Raltegravir potassium, MK-518, D07133 as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain 871038-72-1, Isentress, Raltegravir potassium, MK-518, D07133 and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a 871038-72-1, Isentress, Raltegravir potassium, MK-518, D07133 NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of 871038-72-1, Isentress, Raltegravir potassium, MK-518, D07133 suppliers with NDC on PharmaCompass.
871038-72-1, Isentress, Raltegravir potassium, MK-518, D07133 Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of 871038-72-1, Isentress, Raltegravir potassium, MK-518, D07133 GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right 871038-72-1, Isentress, Raltegravir potassium, MK-518, D07133 GMP manufacturer or 871038-72-1, Isentress, Raltegravir potassium, MK-518, D07133 GMP API supplier for your needs.
A 871038-72-1, Isentress, Raltegravir potassium, MK-518, D07133 CoA (Certificate of Analysis) is a formal document that attests to 871038-72-1, Isentress, Raltegravir potassium, MK-518, D07133's compliance with 871038-72-1, Isentress, Raltegravir potassium, MK-518, D07133 specifications and serves as a tool for batch-level quality control.
871038-72-1, Isentress, Raltegravir potassium, MK-518, D07133 CoA mostly includes findings from lab analyses of a specific batch. For each 871038-72-1, Isentress, Raltegravir potassium, MK-518, D07133 CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
871038-72-1, Isentress, Raltegravir potassium, MK-518, D07133 may be tested according to a variety of international standards, such as European Pharmacopoeia (871038-72-1, Isentress, Raltegravir potassium, MK-518, D07133 EP), 871038-72-1, Isentress, Raltegravir potassium, MK-518, D07133 JP (Japanese Pharmacopeia) and the US Pharmacopoeia (871038-72-1, Isentress, Raltegravir potassium, MK-518, D07133 USP).

