


API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
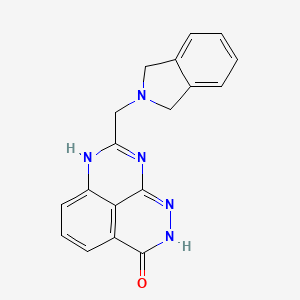

1. 8-(isoindolin-2-ylmethyl)-2,9-dihydro-3h-pyridazino(3,4,5-de)quinazolin-3-one
2. E-7449
3. E7449
4. Parp Inhibitor 2x-121
5. Stenoparib
1. 1140964-99-3
2. E7449
3. E-7449
4. Stenoparib
5. Parp Inhibitor 2x-121
6. Unii-9x5a2qia7c
7. 9x5a2qia7c
8. Cid 25267262
9. 2x-121
10. 11-(1,3-dihydroisoindol-2-ylmethyl)-2,3,10,12-tetrazatricyclo[7.3.1.05,13]trideca-1(12),5(13),6,8,10-pentaen-4-one
11. 3h-pyridazino(3,4,5-de)quinazolin-3-one, 8-((1,3-dihydro-2h-isoindol-2-yl)methyl)-1,2-dihydro-
12. Stenoparib [who-dd]
13. Schembl3886731
14. Chembl3644587
15. Schembl22023558
16. Bcp19934
17. Ex-a1282
18. Nsc783107
19. Nsc798215
20. S8419
21. Zinc59277233
22. Ccg-267668
23. Cs-3479
24. Nsc-783107
25. Nsc-798215
26. Sb16888
27. 8-(1,3-dihydro-isoindol-2-ylmethyl)-2,9-dihydro-1,2,7,9-tetraaza-phenalen-3-one
28. Ac-36842
29. Hy-12418
30. A909458
31. Q27273339
32. 8-(isoindolin-2-ylmethyl)-2,9-dihydro-3h-pyridazino[3,4,5-de]quinazolin-3-one
| Molecular Weight | 317.3 g/mol |
|---|---|
| Molecular Formula | C18H15N5O |
| XLogP3 | 1.1 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 2 |
| Exact Mass | 317.12766012 g/mol |
| Monoisotopic Mass | 317.12766012 g/mol |
| Topological Polar Surface Area | 69.1 Ų |
| Heavy Atom Count | 24 |
| Formal Charge | 0 |
| Complexity | 586 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |


