


API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
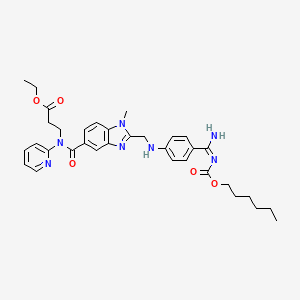

1. Ethyl N-[(2-{[(4-{n'-[(hexyloxy)carbonyl]carbamimidoyl}phenyl)amino]methyl}-1-methyl-1h-benzimidazol-5-yl)carbonyl]-n-pyridin-2-yl-beta-alaninate
2. Pradaxa (tn)
3. Dsstox_cid_31470
4. Dsstox_rid_97355
5. Dsstox_gsid_57681
6. Schembl505829
7. Dabigatran Etexilate (usan/inn)
8. Tox21_113924
9. Bdbm50432209
10. S2154
11. Stl450990
12. Stl483396
13. Ncgc00262929-01
14. Cas-211915-06-9
15. D07144
16. Ab01274780-01
17. Ab01274780_02
18. A815191
19. 3-[[[2-[[4-[(e)-amino-[hexoxy(oxo)methyl]iminomethyl]anilino]methyl]-1-methyl-5-benzimidazolyl]-oxomethyl]-(2-pyridinyl)amino]propanoic Acid Ethyl Ester
20. Ethyl 3-[[2-[[[4-[(e)-n'-hexoxycarbonylcarbamimidoyl]phenyl]amino]methyl]-1-methyl-benzimidazol-5-yl]carbonyl-pyridin-2-yl-amino]propanoate
21. Ethyl N-[(2-{[(4-{n-[(hexyloxy)carbonyl]carbamimidoyl}phenyl)amino]methyl}-1-methyl-1h-benzimidazol-5-yl)carbonyl]-n-pyridin-2-yl-beta-alaninate
| Molecular Weight | 627.7 g/mol |
|---|---|
| Molecular Formula | C34H41N7O5 |
| XLogP3 | 5.7 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 17 |
| Exact Mass | 627.31691743 g/mol |
| Monoisotopic Mass | 627.31691743 g/mol |
| Topological Polar Surface Area | 154 Ų |
| Heavy Atom Count | 46 |
| Formal Charge | 0 |
| Complexity | 1000 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Pradaxa 75 mg
- Primary prevention of venous thromboembolic events in adult patients who have undergone elective total hip replacement surgery or total knee replacement surgery.
Pradaxa 110 mg
- Primary prevention of venous thromboembolic events in adult patients who have undergone elective total hip replacement surgery or total knee replacement surgery.
- Prevention of stroke and systemic embolism in adult patients with non-valvular atrial fibrillation (NVAF), with one or more risk factors, such as prior stroke or transient ischemic attack (TIA); age 75 years; heart failure (NYHA Class II); diabetes mellitus; hypertension.
- Treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE), and prevention of recurrent DVT and PE in adults.
Pradaxa 150 mg
- Prevention of stroke and systemic embolism in adult patients with non-valvular atrial fibrillation (NVAF), with one or more risk factors, such as prior stroke or transient ischemic attack (TIA); age 75 years; heart failure (NYHA Class II); diabetes mellitus; hypertension.
- Treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE), and prevention of recurrent DVT and PE in adults.
B01AE07


