



API Suppliers

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
Other Certificates
0
Other Suppliers
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
Other Listed Suppliers
0
0
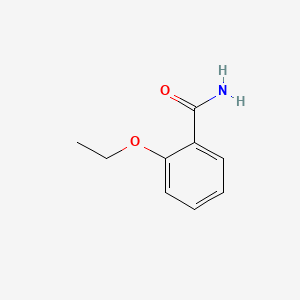

1. 2-ethoxy-benzamide
2. 2-ethoxybenzamide
3. O-ethoxybenzamide
1. 2-ethoxybenzamide
2. 938-73-8
3. O-ethoxybenzamide
4. Benzamide, 2-ethoxy-
5. Etenzamide
6. Ethbenzamide
7. Ethenzamid
8. Etamide
9. Anovigam
10. Ethosalicyl
11. Lucamide
12. Pirosolvina
13. Etosalicil
14. Etosalicyl
15. Katagrippe
16. Protopyrin
17. Trancalgyl
18. Etocil
19. Eusal
20. Benzamide, O-ethoxy-
21. Nsc 28787
22. H.p. 209
23. Nsc-28787
24. L929zck4bf
25. J3.352i
26. Ncgc00091616-01
27. Ethenzamide 100 Microg/ml In Acetonitrile
28. Dsstox_cid_581
29. Dsstox_rid_75671
30. Dsstox_gsid_20581
31. Etenzamide [dcit]
32. Etenzamida
33. Ethenzamidum
34. Etenzamida [inn-spanish]
35. Ethenzamidum [inn-latin]
36. 2-eethoxybenzamide
37. Cas-938-73-8
38. Ccris 9124
39. Einecs 213-346-4
40. Unii-l929zck4bf
41. Brn 2208582
42. Ethanzamide
43. Ethenzamide [inn:ban:jan]
44. Orthoethoxybenzamide
45. Ethenzamide (tn)
46. 2-ethoxybenzoylamide
47. Mfcd00007977
48. Ethenzamide [mi]
49. Ethenzamide [inn]
50. Ethenzamide [jan]
51. 2-ethoxybenzamide, 97%
52. Wln: Zvr Bo2
53. Ethenzamide (jp17/inn)
54. Ethenzamide [mart.]
55. Schembl25624
56. Ethenzamide [who-dd]
57. 4-10-00-00175 (beilstein Handbook Reference)
58. Mls002302987
59. Zinc1384
60. Chembl1483877
61. Dtxsid4020581
62. Chebi:31564
63. Hms3039j16
64. Ethenzamide 1.0 Mg/ml In Methanol
65. Hy-b1428
66. Nsc28787
67. Tox21_111156
68. Tox21_200025
69. S4524
70. Stk105005
71. Akos003280312
72. Tox21_111156_1
73. Ccg-213844
74. Cs-4916
75. Db13544
76. Ks-5320
77. Ncgc00091616-02
78. Ncgc00091616-03
79. Ncgc00257579-01
80. Ac-11991
81. Smr001307304
82. Db-057442
83. E0222
84. Ft-0612206
85. D01466
86. D70657
87. Ab01010349_03
88. A844727
89. Q553324
90. Sr-01000877236
91. Q-201075
92. Sr-01000877236-2
93. Z54953371
| Molecular Weight | 165.19 g/mol |
|---|---|
| Molecular Formula | C9H11NO2 |
| XLogP3 | 1.2 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 3 |
| Exact Mass | 165.078978594 g/mol |
| Monoisotopic Mass | 165.078978594 g/mol |
| Topological Polar Surface Area | 52.3 Ų |
| Heavy Atom Count | 12 |
| Formal Charge | 0 |
| Complexity | 159 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anti-Inflammatory Agents, Non-Steroidal
Anti-inflammatory agents that are non-steroidal in nature. In addition to anti-inflammatory actions, they have analgesic, antipyretic, and platelet-inhibitory actions. They act by blocking the synthesis of prostaglandins by inhibiting cyclooxygenase, which converts arachidonic acid to cyclic endoperoxides, precursors of prostaglandins. Inhibition of prostaglandin synthesis accounts for their analgesic, antipyretic, and platelet-inhibitory actions; other mechanisms may contribute to their anti-inflammatory effects. (See all compounds classified as Anti-Inflammatory Agents, Non-Steroidal.)
N - Nervous system
N02 - Analgesics
N02B - Other analgesics and antipyretics
N02BA - Salicylic acid and derivatives
N02BA07 - Ethenzamide


