



API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
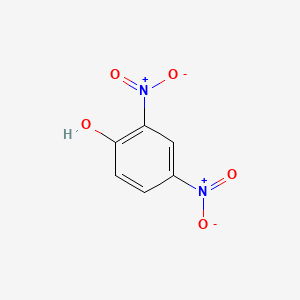

1. 2,4 Dinitrophenol
2. 2,4-dnp
1. 51-28-5
2. Aldifen
3. Phenol, 2,4-dinitro-
4. Nitrophen
5. Nitrophene
6. Fenoxyl Carbon N
7. Solfo Black B
8. Sulphur Black 1
9. Tertrosulphur Pbr
10. Alpha-dinitrophenol
11. Dinofan
12. Dinitrophenol
13. Solfo Black G
14. 1-hydroxy-2,4-dinitrobenzene
15. 2,4-dnp
16. Nitro Kleenup
17. Chemox Pe
18. Solfo Black Bb
19. Solfo Black Sb
20. Dinitrofenolo
21. .alpha.-dinitrophenol
22. Tertrosulphur Black Pb
23. Solfo Black 2b Supra
24. 2,4-dinitrofenol
25. Maroxol-50
26. 1326-82-5
27. Rcra Waste Number P048
28. Nsc 1532
29. Ek 102
30. Chemox
31. Mitcal
32. X 32
33. 2,4-dinitro-phenol
34. Tertrosulfur Pbr
35. Chebi:42017
36. Nsc-1532
37. Mfcd00007115
38. Phenol, .alpha.-dinitro-
39. Q13sks21mn
40. 1,3-dinitro-4-hydroxybenzene
41. 2,4-dinitrophenol (h2o W/w 18% Max)
42. Dsstox_cid_523
43. Osmoplastic-r
44. Osmotox-plus
45. Tertrosulfur Black Pb
46. Dsstox_rid_75640
47. Dsstox_gsid_20523
48. Nitrophen (van)
49. Dnf
50. Nitrophene (van)
51. 2,4-dinitrophenol (wetted With Up To 20per Cent Water)
52. Camello Mosquito Coils
53. Caswell No. 392
54. Dinitrofenolo [italian]
55. Phenol, Alpha-dinitro-
56. Cas-51-28-5
57. 2,4-dinitrofenol [dutch]
58. 1'alpha-2,4-dinitrophenol
59. Cobra Salts (impregna Salts)
60. Shirakiku Brand Mosquito Coils
61. Ccris 3102
62. Hsdb 529
63. Einecs 200-087-7
64. Rcra Waste No. P048
65. Unii-q13sks21mn
66. Epa Pesticide Chemical Code 037509
67. Ai3-01535
68. Spectrum_001934
69. Specplus_000938
70. Spectrum2_000673
71. Spectrum3_001694
72. Spectrum4_000913
73. Spectrum5_001670
74. Wln: Wnr Bq Enw
75. Epitope Id:112424
76. Ec 200-087-7
77. 2,4-dinitrophenol, Wetted
78. Dinitrophenol, Wetted
79. Schembl77643
80. Bspbio_003248
81. Kbiogr_001406
82. Kbioss_002480
83. Spectrum330008
84. Mls001066358
85. Bidd:er0505
86. Dinitrophenol [mart.]
87. Divk1c_007034
88. Spbio_000765
89. Dinitrophenol [who-dd]
90. Chembl273386
91. Sr-1c5
92. Dtxsid0020523
93. Schembl13973810
94. Kbio1_001978
95. Kbio2_002473
96. Kbio2_005041
97. Kbio2_007609
98. Kbio3_002468
99. 2,4-dinitrophenol [mi]
100. Nsc1532
101. Hms1923k21
102. Hms2233h07
103. Hms3372p06
104. Hms3867n13
105. 2,4-dinitrophenol [hsdb]
106. Tox21_201462
107. Tox21_300030
108. Ccg-39730
109. Nsc791780
110. Stk397797
111. Zinc12358776
112. Akos000118988
113. Akos015912556
114. Db04528
115. Nsc-791780
116. Ncgc00091778-01
117. Ncgc00091778-02
118. Ncgc00091778-03
119. Ncgc00091778-04
120. Ncgc00091778-05
121. Ncgc00091778-06
122. Ncgc00254114-01
123. Ncgc00259013-01
124. As-39888
125. Smr000471854
126. D0109
127. 2,4-dinitrophenol (wetted With 15 % Water)
128. C02496
129. Ab00053210_08
130. 2,4-dinitrophenol, Saj Special Grade, >=98.0%
131. Af-936/31262030
132. Q209226
133. 2,4-dinitrophenol, Moistened With Water, >=98.0%
134. W-105909
135. 2,4-dinitrophenol, 96%, Stab. With Min. 15% Water
136. 2,4-dinitrophenol, Pestanal(r), Analytical Standard
137. Brd-k21910317-001-02-4
138. Brd-k21910317-001-06-5
139. Brd-k21910317-001-12-3
140. 2,4-dinitrophenol, Technical, Moistened With Water, >=97.0% (hplc)
| Molecular Weight | 184.11 g/mol |
|---|---|
| Molecular Formula | C6H4N2O5 |
| XLogP3 | 1.7 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 0 |
| Exact Mass | 184.01202123 g/mol |
| Monoisotopic Mass | 184.01202123 g/mol |
| Topological Polar Surface Area | 112 Ų |
| Heavy Atom Count | 13 |
| Formal Charge | 0 |
| Complexity | 220 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Former use: Early in the 1930's 2,4-dinitrophenol was highly recommended for the treatment of obesity. During the first 15 months following the introduction of the use, an estimated 100,000 persons took the drug for weight reduction ... . Typical treatment regimen for weight control consisted of one capsule containing 75 mg of 2,4-dinitrophenol or 100 mg of the sodium salt taken 3 times daily after meals (2 to 5 mg/kg/day).
National Research Council. Drinking Water & Health, Volume 4. Washington, DC: National Academy Press, 1981., p. 237
Use of 2,4-dinitrophenol as a human dieting aid has produced some cases of agranulocytosis, neuritis, and functional heart damage. ... /Former use/
Horner WD; Arch Ophthamol 27: 1097 (1942)
Coloring Agents
Chemicals and substances that impart color including soluble dyes and insoluble pigments. They are used in INKS; PAINTS; and as INDICATORS AND REAGENTS. (See all compounds classified as Coloring Agents.)
Uncoupling Agents
Chemical agents that uncouple oxidation from phosphorylation in the metabolic cycle so that ATP synthesis does not occur. Included here are those IONOPHORES that disrupt electron transfer by short-circuiting the proton gradient across mitochondrial membranes. (See all compounds classified as Uncoupling Agents.)
2,4-DNP appears to be readily absorbed from the respiratory and gastrointestinal tracts. Some evidence suggests significant absorption through the skin as well. 2,4-DNP is relatively lipophilic with a pKa of 4.09 and therefore is likely to be rapidly absorbed by passive diffusion of the un-ionized form from acidic compartments like the stomach. Another factor favoring the rapid absorption of 2,4-DNP is its small molecular weight (184.1). Molecules with a molecular weight below 600 daltons can permeate cell membranes through aqueous channels regardless of their ionization state.
U.S. Dept Health & Human Services/Agency for Toxic Substances & Disease Registry; Toxicological Profile for Dinitrophenols p.91 (August 1995) Toxic Profiles 64. Available from, as of September 16, 2010: https://www.atsdr.cdc.gov/toxprofiles/index.asp
2,4-DNP appears to be metabolized to less toxic metabolites (primarily aminonitrophenols) that are excreted in the urine. The mechanism of excretion may be passive diffusion, in part, although there is some evidence from one in vitro study of active secretion of 2,4-DNP by the renal organic acid transport process.
U.S. Dept Health & Human Services/Agency for Toxic Substances & Disease Registry; Toxicological Profile for Dinitrophenols pp.91-2 (August 1995) Toxic Profiles 64. Available from, as of September 16, 2010: https://www.atsdr.cdc.gov/toxprofiles/index.asp
It is readily absorbed from skin and through respiratory tract and GI tract.
Grant, W.M. Toxicology of the Eye. 3rd ed. Springfield, IL: Charles C. Thomas Publisher, 1986., p. 359
Studies of blood aqueous barrier & differences of penetrability of 2,4-dinitrophenol into eye from blood in different species ... have shown that dinitrophenol gets into aqueous humor more readily & reaches higher concn in ducks that develop cataracts than ... rabbits that do not. Also, in immature rabbits, in which cataracts can be produced, dinitrophenol entered aqueous humor more readily than in mature rabbits which did not develop cataracts.
Grant, W.M. Toxicology of the Eye. 3rd ed. Springfield, IL: Charles C. Thomas Publisher, 1986., p. 359
For more Absorption, Distribution and Excretion (Complete) data for 2,4-Dinitrophenol (10 total), please visit the HSDB record page.
2,4-DNP and two of its metabolites, 2-amino-4-nitrophenol and 4-amino-2-nitrophenol, were monitored in plasma of mice at 0.5, 1, 2, 4, 6, 9, 12, 24, 48, and 96 hours following a single gavage dose of 22.5 mg/kg using a highly specific capillary GC/MS technique. /It was/ concluded that the amount of 2-amino-4-nitrophenol formed was 7.9 times the amount of 4-amino-2-nitrophenol, and that 50% of 2,4-DNP elimination involved direct conversion to these two compounds. Plasma concentrations of these two metabolites reached their highest levels within the first half hour after dosing, indicating rapid metabolism. The results demonstrate that 2-amino-4- nitrophenol is the major circulating metabolite of 2,4-DNP and that 4-amino-2-nitrophenol is also a significant circulating metabolite.
U.S. Dept Health & Human Services/Agency for Toxic Substances & Disease Registry; Toxicological Profile for Dinitrophenols p.85 (August 1995) Toxic Profiles 64. Available from, as of September 16, 2010: https://www.atsdr.cdc.gov/toxprofiles/index.asp
/An/ investigation of the in vitro metabolism of 2,4-DNP by rat liver homogenates found that, under optimal pH and cofactor levels, 81% of the 2,4-DNP was metabolized. 2-Amino-4-nitrophenol accounted for 75%, 4-amino-2-nitrophenol for 23%, and 2,4-diaminophenol for approx 1% of the total amine metabolites produced. Even under suboptimal conditions, 2-amino-4-nitrophenol was the predominant metabolite.
U.S. Dept Health & Human Services/Agency for Toxic Substances & Disease Registry; Toxicological Profile for Dinitrophenols p.86 (August 1995) Toxic Profiles 64. Available from, as of September 16, 2010: https://www.atsdr.cdc.gov/toxprofiles/index.asp
The distribution of enzyme activity was analyzed in subcellular fractions: nucleic, mitochondrial, microsomal, and cytosol. The maximum activity was found in the cytosol, which is the site of other nitroreductases, although nitroreductases can also be located in microsomes. The properties of nitroreductases have been extensively studied for the reduction of p-nitrobenzoic acid. Two separate enzyme systems are involved, one located in the cytosol, and the other in the microsomes. Both forms require the presence of reduced nicotinamide adenine dinucleotides (NADH or NADPH). The cytosolic reducing activity for 2,4-DNP required NADPH, since the activity in both the whole homogenate and in the cytosol was enhanced by adding glucose-6-phosphatase and NADP. The fact that the washed microsomal fraction contained no appreciable activity with 2,4-DNP could be due to the absence of soluble NADPH-generating enzymes, such as a glucose-6-phosphate dehydrogenase. Oxygen partially inhibited the formation of the aminonitrophenols. This inhibition is consistent with a reoxidation of cofactors FADH2 or NADPH in the presence of oxygen. Reduction of (14)C-2,4-DNP to 2-amino-4-nitrophenol and 4-amino-2-nitrophenol by rat liver homogenates was not affected by the addition of p-nitrobenzoic acid, suggesting that different nitroreductases are involved. However, p-nitrophenol, o-nitrophenol, and 2,4-dinitro-6-sec-butylphenol inhibited the reduction of 2,4-DNP. The reduction was competitively inhibited by o-nitrophenol and noncompetitively inhibited by p-nitrophenol and 2,4-dinitro-6-sec-butylphenol. These results indicate separate metabolic pathways for 2,4-DNP and p-nitrobenzoic acid. The competitive inhibition by o-nitrophenol, however, suggests that 2,4-DNP and o-nitrophenol compete for the same active site on the nitroreductase, while the noncompetitive inhibition by the other two nitro compounds suggests binding at different sites on the enzyme. Limited information indicates that 2,4-DNP may also be conjugated to glucuronic acid or sulfate in the liver and then be excreted in the urine.
U.S. Dept Health & Human Services/Agency for Toxic Substances & Disease Registry; Toxicological Profile for Dinitrophenols pp.86,88 (August 1995) Toxic Profiles 64. Available from, as of September 16, 2010: https://www.atsdr.cdc.gov/toxprofiles/index.asp
In urine of /sc/ treated rabbits, dinitrophenol glucuronide, 2-aminonitrophenol & its ether sulfate were found. In vitro, but not in vivo, studies indicated presence of 4-aminonitrophenol. Latter was unstable ... .
Menzie, C.M. Metabolism of Pesticides. U.S. Department of the Interior, Bureau of Sport Fisheries and Wildlife, Publication 127. Washington, DC: U.S. Government Printing Office, 1969., p. 172
For more Metabolism/Metabolites (Complete) data for 2,4-Dinitrophenol (8 total), please visit the HSDB record page.
Mice given 20 mg/kg ip of 2,4-dinitrophenol exhibited a half-life for elimination of 54.0 minutes. Rats given 20 mg/kg ip ... exhibited a half-life of 225.0 minutes. /From table/
Ambient Water Quality Criteria Doc: Nitrophenols p.C-37 (1980) EPA 440/5-80-063
2,4-DNP was demonstrated to be an uncoupler of oxidative phosphorylation in an in vitro study. 2,4-DNP prevented phosphorylation without affecting, or with a slight stimulation of, oxidation. During the Krebs cycle, 2,4-DNP uncouples oxidative phosphorylation from electron transport by carrying protons across the inner mitochondrial membrane, thereby dissipating the pH gradient and membrane electrochemical potential and preventing the formation of adenosine triphosphate (ATP). During this uncoupling, electron transport from NADH to oxygen proceeds normally, but the energy produced, which is normally stored in high-energy phosphate bonds in ATP, is released as heat. The small amount of ATP produced directly from glycolysis is not affected. In vitro studies further demonstrated and investigated the ability of 2,4-DNP to uncouple oxidative phosphorylation. 2,4-DNP increased the rate of oxygen uptake and changed the electron microscopic appearance of rat liver mitochondria in vitro from an expanded configuration to a condensed state, with the ultrastructural change occurring in concert with the functional changes of uncoupling. All energy-dependent biochemical processes thus are likely to be affected. Many of the clinical observations of 2,4-DNP toxicity, such as elevated basal metabolic rate or oxygen consumption, elevated respiration and pulse rates, increased perspiration, increased body temperature in humans and animals are related to the uncoupling of oxidative phosphorylation. When heat production exceeds the organism's capacity to dissipate heat, fatal hyperthermia may result
U.S. Dept Health & Human Services/Agency for Toxic Substances & Disease Registry; Toxicological Profile for Dinitrophenols p.92 (August 1995) Toxic Profiles 64. Available from, as of September 16, 2010: https://www.atsdr.cdc.gov/toxprofiles/index.asp
The uncoupling of mitochondrial electron transport from oxidative phosphorylation with resultant decreased production of ATP by 2,4-DNP appears to be related to the cataractogenesis of 2,4-DNP. The lens epithelium is the chief source of available energy for the lens. In most animals, the energy needs are met principally by anaerobic glycolysis, and <30% by oxidative phosphorylation. In incubated bovine lenses, oxygen was not necessary for maintaining sodium levels in the presence of glucose, suggesting anaerobic respiration in the lens. Energy evolved from the breakdown of ATP by Na+/K+-activated ATPase is required for the transport of these cations across the lens epithelium to maintain proper ionic balance. Sodium is actively transported from the lens to the aqueous humor, while potassium is actively transported in the reverse direction. Interference with this active transport mechanism across the lens epithelium can result in increased sodium in the lens, disruption of the ionic balance between the lens and aqueous humor, and subsequent cataract formation. An in vitro study with rabbit lenses also demonstrated that 2,4-DNP does not cause calcium-induced cataracts by interfering with active transport of calcium from the lens, because the energy for calcium transport is derived from anaerobic glycolysis and not oxidative phosphorylation.
U.S. Dept Health & Human Services/Agency for Toxic Substances & Disease Registry; Toxicological Profile for Dinitrophenols pp.92-3 (August 1995) Toxic Profiles 64. Available from, as of September 16, 2010: https://www.atsdr.cdc.gov/toxprofiles/index.asp
Transthyretin (TTR), a homotetrameric thyroxine transport protein found in the plasma and cerebrospinal fluid, circulates normally as a innocuous soluble protein. In some individuals, TTR polymerizes to form insoluble amyloid fibrils. TTR amyloid fibril formation and deposition have been associated with several diseases like familial amyloid polyneuropathy and senile systemic amyloidosis. Inhibition of the fibril formation is considered a potential strategy for the therapeutic intervention. The effect of small water-soluble, hydrophobic ligand 2,4-dinitrophenol (2,4-DNP) on TTR amyloid formation has been tested. 2,4-DNP binds to TTR both at acidic and physiological pH, as shown by the quenching of TTR intrinsic fluorescence. Interestingly, 2,4-DNP not only binds to TTR at acidic pH but also inhibits amyloid fibril formation as shown by the light scattering and Congo red-binding assay. Inhibition of fibril formation by 2,4-DNP appears to be through the stabilization of TTR tetramer upon binding to the protein, which includes active site. These findings may have implications for the development of mechanism based small molecular weight compounds as therapeutic agents for the prevention/inhibition of the amyloid diseases.
PMID:11913969 Raghu P et al; Arch Biochem Biophys 400 (1): 43-7 (2002)
Systemic deposition of transthyretin (TTR) amyloid fibrils is always observed in familial amyloidotic polyneuropathy, senile systemic amyloidosis and familial amyloidotic cardiomyopathy patients. Destabilization of the molecule leads to a cascade of events which result in fibril formation. The destabilization of a native protein with consequent conformational changes appears to be a common link in several human amyloid diseases. Intensive research has been directed towards finding small molecules that could work as therapeutic agents for the prevention/inhibition of amyloid diseases through stabilization of the native fold of the potentially amyloidogenic protein. This work provides insight into the structural determinants of the highly stabilizing effects of 2,4-dinitrophenol on wild-type TTR. It is also shown that similar interactions are established between this molecule and two highly amyloidogenic TTR variants: TTR L55P and TTR Y78F. In the three crystal complexes, 2,4-dinitrophenol occupies the two hormone-binding sites of the TTR tetramer. As a result of 2,4-dinitrophenol binding, the two dimers in the TTR tetramer become closer, increasing the stability of the protein. The three-dimensional structures now determined allow a comprehensive description of key interactions between transthyretin and 2,4-dinitrophenol, a small compound that holds promise as a template for the design of a therapeutical drug for amyloid diseases.
PMID:16627944 Morais-de-Sa E et al; Acta crystallographica. Sect D. Biological Crystallography 62 (Pt 5): 512-9 (2006)
For more Mechanism of Action (Complete) data for 2,4-Dinitrophenol (24 total), please visit the HSDB record page.


