


API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
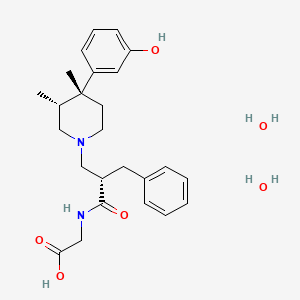

1. Adl 8-2698
2. Adl8-2698
3. Alvimopan
4. Alvimopan Anhydrous
5. Anhydrous Alvimopan
6. Ly 246736
7. Ly-246736
8. Ly246736
9. Trans-3,4-dimethyl-4-(3-hydroxyphenyl) Piperidine
1. Alvimopan Dihydrate
2. 170098-38-1
3. Alvimopan Hydrate
4. Alvimopan (dihydrate)
5. Ly 246736 Dihydrate
6. Alvimopan [usan]
7. Ly-246736 Dihydrate
8. 677c126aet
9. Alvimopan (usan)
10. Adl-8-2698
11. 2-[[(2s)-2-benzyl-3-[(3r,4r)-4-(3-hydroxyphenyl)-3,4-dimethyl-1-piperi Dyl]propanoyl]amino]acetic Acid Dihydrate
12. 2-[[(2s)-2-benzyl-3-[(3r,4r)-4-(3-hydroxyphenyl)-3,4-dimethylpiperidin-1-yl]propanoyl]amino]acetic Acid;dihydrate
13. (((2s)-2-(((3r,4r)-4-(3-hydroxyphenyl)-3,4-dimethylpiperidin-1-yl)methyl)-3-phenylpropanoyl)amino)acetic Acid Dihydrate
14. 2-[[(2s)-2-benzyl-3-[(3r,4r)-4-(3-hydroxyphenyl)-3,4-dimethylpiperidin-1-yl]propanoyl]amino]acetic Acid,dihydrate
15. Ly246736
16. Ly-246736
17. Entrareg
18. Alvimopan [usan:inn:ban]
19. Unii-677c126aet
20. Alvimopandihydrate
21. 156053-89-3 (anhydrous)
22. Entereg (tn)
23. Alvimopan [hsdb]
24. Alvimopan [vandf]
25. Alvimopan 2-hydrate
26. Schembl1477341
27. Alvimopan [orange Book]
28. Alvimopan Dihydrate [mi]
29. Dtxsid40168794
30. Hms3886i18
31. Alvimopan Dihydrate [mart.]
32. Bcp10120
33. Sb-767905
34. Hy-76657a
35. S5701
36. Akos015899533
37. Ac-8813
38. Am84590
39. Ccg-269339
40. Cs-1540
41. As-75035
42. Ba164152
43. Glycine, N-((2s)-2-(((3r,4r)-4-(3-hydroxyphenyl)-3,4-dimethyl-1-piperidinyl)methyl)-1-oxo-3-phenylpropyl)-, Dihydrate
44. Glycine, N-(2-((4-(3-hydroxyphenyl)-3,4-dimethyl-1-piperidinyl)methyl)-1-oxo-3-phenylpropyl)-, Dihydrate, (3r-(1(s*),3alpha,4alpha))-
45. Glycine, N-(2-((4-(3-hydroxyphenyl)-3,4-dimethyl-1-piperidinyl)methyl)-1-oxo-3-phenylpropyl)-,dihydrate, (3r-(1(s*),3-alpha,4-alpha))-
46. D02878
47. D70720
48. 098a381
49. A882054
50. (2s, 3r, 4r)[[2-[[4-(3-hydroxyphenyl)-3,4-dimethyl-1-piperidinyl ]methyl]-1-oxo-3-phenylpropyl]-amino]acetic Acid Dihydrate
51. 2-((s)-2-benzyl-3-((3r,4r)-4-(3-hydroxyphenyl)-3,4-dimethylpiperidin-1-yl)propanamido)acetic Acid Dihydrate
52. 2-[(2s)-2-{[(3r,4r)-4-(3-hydroxyphenyl)-3,4-dimethylpiperidin-1-yl]methyl}-3-phenylpropanamido]acetic Acid Dihydrate
53. 2-[(2s)-2-benzyl-3-[(3r,4r)-4-(3-hydroxyphenyl)-3,4-dimethylpiperidin-1-yl]propanamido]acetic Acid Dihydrate
54. Glycine, N-(2-((4-(3-hydroxyphenyl)-3,4-dimethyl-1-piperidinyl)methyl)-1-oxo-3-phenylpropyl)-, Dihydrate, (3r-(1(s*),3.alpha.,4.alpha.))-
| Molecular Weight | 460.6 g/mol |
|---|---|
| Molecular Formula | C25H36N2O6 |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 8 |
| Exact Mass | 460.25733687 g/mol |
| Monoisotopic Mass | 460.25733687 g/mol |
| Topological Polar Surface Area | 91.9 Ų |
| Heavy Atom Count | 33 |
| Formal Charge | 0 |
| Complexity | 606 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 3 |
Gastrointestinal Agents
Drugs used for their effects on the gastrointestinal system, as to control gastric acidity, regulate gastrointestinal motility and water flow, and improve digestion. (See all compounds classified as Gastrointestinal Agents.)


