



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
Other Certificates
0
Other Suppliers

USA (Orange Book)

Europe

Canada

Australia
0

South Africa
Uploaded Dossiers
U.S. Medicaid
0
Annual Reports
0
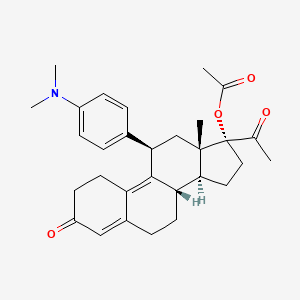

1. (11beta)-17-(acetyloxy)-11-(4-(dimethylamino)phenyl)-19-norpregna-4,9-diene-3,20-dione
2. Ella Norpregnadiene
3. Esmya
1. 126784-99-4
2. Cdb-2914
3. Ella
4. Ellaone
5. Cdb 2914
6. Hrp 2000
7. Ulipristal Acetate [usan]
8. Va2914
9. (8s,11r,13s,14s,17r)-17-acetyl-11-(4-(dimethylamino)phenyl)-13-methyl-3-oxo-2,3,6,7,8,11,12,13,14,15,16,17-dodecahydro-1h-cyclopenta[a]phenanthren-17-yl Acetate
10. (11beta)-17-(acetyloxy)-11-[4-(dimethylamino)phenyl]-19-norpregna-4,9-diene-3,20-dione
11. Yf7v70n02b
12. Chebi:71025
13. 17-acetoxy-11-(4-n,n-dimethylaminophenyl)pregna-4,9-diene-3,20-dione
14. Pgl-4001
15. Va-2914
16. Rti-3021-012
17. Ulipristal (acetate)
18. (11b)-17-(acetyloxy)-11-[4-(dimethylamino)phenyl]-19-norpregna-4,9-diene-3,20-dione
19. (11beta)-17-(acetyloxy)-11-(4-(dimethylamino)phenyl)-19-norpregna-4,9-diene-3,20-dione
20. (11beta,17alpha)-17-acetyl-11-[4-(dimethylamino)phenyl]-3-oxoestra-4,9-dien-17-yl Acetate
21. 17beta-acetyl-11beta-[4-(dimethylamino)phenyl]-3-oxoestra-4,9-dien-17alpha-yl Acetate
22. Unii-yf7v70n02b
23. Hrp-2000
24. Ulipristal Acet
25. Mfcd00899035
26. 4oar
27. Ulipristal-acetate
28. Ella Norpregnadiene
29. [(8s,11r,13s,14s,17r)-17-acetyl-11-[4-(dimethylamino)phenyl]-13-methyl-3-oxo-1,2,6,7,8,11,12,14,15,16-decahydrocyclopenta[a]phenanthren-17-yl] Acetate
30. Cbd 2914
31. Ella (tn)
32. Rti 3021-012
33. C17 Epi Ulipristal Acetate
34. Schembl544957
35. Chembl260538
36. Gtpl7460
37. Ulipristal Acetate [mi]
38. Ulipristal Acetate (jan/usan)
39. Ulipristal Acetate [jan]
40. Dtxsid30155294
41. Ta[a]phenanthren-17-yl] Acetate
42. Ulipristal Acetate [vandf]
43. Ulipristal Acetate [mart.]
44. Ulipristal Acetate [who-dd]
45. Zinc3920657
46. Bdbm50375424
47. Ulipristal Acetate, >=98% (hplc)
48. Akos026750526
49. Ccg-269500
50. Cs-1157
51. Ulipristal Acetate [orange Book]
52. Ncgc00378913-02
53. [(8s,11r,13s,14s,17r)-17-acetyl-11-(4-dimethylaminophenyl)-13-methyl-3-oxo-1,2,6,7,8,11,12,14,15,16-decahydrocyclopenta[a]phenanthren-17-yl] Acetate
54. As-73950
55. Bu161520
56. Hy-16508
57. U0102
58. C72119
59. D09687
60. Ab01566874_01
61. 784a994
62. Q975059
63. J-005436
64. Brd-k64381438-001-03-8
65. Z2216208644
66. 5-(hydroxymethyl)-alpha,alpha,alpha,alpha-tetramethyl-1,3-benzenediacetonitrile
67. (10s,11s,14r,15s,17r)-14-acetyl-17-[4-(dimethylamino)phenyl]-15-methyl-5-oxotetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadeca-1,6-dien-14-yl Acetate
68. (11.beta.)-17-(acetyloxy)-11-(4-(dimethylamino)phenyl)-19-norpregna-4,9-diene-3,20-dione
69. (11alpha,13alpha,17beta)-17-acetyl-11-[4-(dimethylamino)phenyl]-3-oxoestra-4,9-dien-17-yl Acetate
70. (1r,3as,3bs,10r,11as)-1-acetyl-10-[4-(dimethylamino)phenyl]-11a-methyl-7-oxo-1h,2h,3h,3ah,3bh,4h,5h,7h,8h,9h,10h,11h,11ah-cyclopenta[a]phenanthren-1-yl Acetate
71. (8s,11r,13s,14s,17r)-17-acetyl-11-(4-(dimethylamino)phenyl)-13-methyl-3-oxo-2,3,6,7,8,11,12,13,14,15,16,17-dodecahydro-1h-cyclopenta[a]phenanthren-17-ylacetate
72. [(8s,11r,13s,14s,17r)-17-acetyl-11-[4-(dimethylamino)phenyl]-13-methyl-3-oxo-1,2,6,7,8,11,12,14,15,16-decahydrocyclopen
73. 17.alpha.-acetoxy-11.beta.-(4-dimethylaminophenyl)-19-norpregna-4,9-dien-3,20-dione
74. 19-norpregna-4,9-diene-3,20-dione, 17-(acetyloxy)-11-(4-(dimethylamino)phenyl)-, (11beta)-
75. C17 Isomer Ulipristal Acetate; (10s,11s,14s,15s,17r)-14-acetyl-17-[p-(dimethylamino)phenyl]-15-methyl-5-oxotetracyclo[8.7.0.02,7.011,15]heptadeca-1,6-dien-14-yl Acetate
76. Cbd 2914; Va 2914; (11b)-17-(acetyloxy)-11-[4-(dimethylamino)phenyl]-19-norpregna-4,9-diene-3,20-dione
| Molecular Weight | 475.6 g/mol |
|---|---|
| Molecular Formula | C30H37NO4 |
| XLogP3 | 3.5 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 5 |
| Exact Mass | 475.27225866 g/mol |
| Monoisotopic Mass | 475.27225866 g/mol |
| Topological Polar Surface Area | 63.7 Ų |
| Heavy Atom Count | 35 |
| Formal Charge | 0 |
| Complexity | 984 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 5 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Ulipristal acetate is indicated for one treatment course of pre-operative treatment of moderate to severe symptoms of uterine fibroids in adult women of reproductive age.
Ulipristal acetate is indicated for intermittent treatment of moderate to severe symptoms of uterine fibroids in adult women of reproductive age who are not eligible for surgery.
Emergency contraception within 120 hours (five days) of unprotected sexual intercourse or contraceptive failure.
Contraception
Leiomyoma of uterus
Contraceptive Agents, Female
Chemical substances or agents with contraceptive activity in females. Use for female contraceptive agents in general or for which there is no specific heading. (See all compounds classified as Contraceptive Agents, Female.)
Contraceptive Agents, Hormonal
Contraceptive agents that act on the ENDOCRINE SYSTEM. (See all compounds classified as Contraceptive Agents, Hormonal.)
G03XB02
G03AD02




