



API Suppliers

US DMFs Filed

CEP/COS Certifications

JDMFs Filed
0
Other Certificates
Other Suppliers

USA (Orange Book)

Europe

Canada

Australia

South Africa
Uploaded Dossiers
U.S. Medicaid
Annual Reports
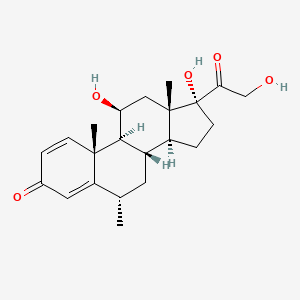

1. 6 Methylprednisolone
2. 6-methylprednisolone
3. Medrol
4. Metipred
5. Urbason
1. 83-43-2
2. Medrol
3. Metilprednisolone
4. Medrone
5. Medrate
6. Urbason
7. 6alpha-methylprednisolone
8. Metilbetasone
9. Dopomedrol
10. Promacortine
11. Besonia
12. Medesone
13. Mesopren
14. Metastab
15. Metrisone
16. Moderin
17. Noretona
18. Solomet
19. Urbasone
20. Wyacort
21. Methylprednisolon
22. Methyleneprednisolone
23. Metilprednisolone [dcit]
24. Delta(1)-6alpha-methylhydrocortisone
25. 1-dehydro-6alpha-methylhydrocortisone
26. Nsc-19987
27. Metilprednisolona
28. Methylprednisolonum
29. Methylprednisolonum [inn-latin]
30. Metilprednisolona [inn-spanish]
31. 6-alpha-methylprednisolone
32. Suprametil
33. Medrol Dosepak
34. Medrol Adt Pak
35. 6alpha-methyl-11beta,17alpha,21-triol-1,4-pregnadiene-3,20-dione
36. Chebi:6888
37. 11beta,17,21-trihydroxy-6alpha-methylpregna-1,4-diene-3,20-dione
38. (6alpha,11beta)-11,17,21-trihydroxy-6-methylpregna-1,4-diene-3,20-dione
39. (6s,8s,9s,10r,11s,13s,14s,17r)-11,17-dihydroxy-17-(2-hydroxyacetyl)-6,10,13-trimethyl-7,8,9,11,12,14,15,16-octahydro-6h-cyclopenta[a]phenanthren-3-one
40. Prednisolone, Methyl-
41. Methylprednisolone, 6-alpha
42. 6alpha-methylprednisolone Base
43. U 7532
44. Mls000028541
45. (6s,8s,9s,10r,11s,13s,14s,17r)-11,17-dihydroxy-17-(2-hydroxyacetyl)-6,10,13-trimethyl-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-3h-cyclopenta[a]phenanthren-3-one
46. Sieropresol
47. Esametone
48. Firmacort
49. Medixon
50. Metrocort
51. Metysolon
52. Nirypan
53. Reactenol
54. Artisone-wyeth
55. X4w7zr7023
56. Meprdl
57. Predni N Tablinen
58. J3.872e
59. Medlone 21
60. Smr000058330
61. Dsstox_cid_3300
62. Pregna-1,4-diene-3,20-dione, 11,17,21-trihydroxy-6-methyl-, (6a,11b)-
63. Prednol- L
64. Dsstox_rid_76965
65. Dsstox_gsid_23300
66. Depo-medrol (acetate)
67. Summicort
68. Cas-83-43-2
69. Prednisolone, 6alpha-methyl-
70. Hsdb 3127
71. Mls002638721
72. Einecs 201-476-4
73. Prednisolone, 6.alpha.-methyl-
74. Brn 2340300
75. 6-alpha-methylprednisolone 100 Microg/ml In Acetonitrile
76. 11beta,17alpha,21-trihydroxy-6alpha-methyl-1,4-pregnadiene-3,20-dione
77. Delta(sup 1)-6-alpha-methylhydrocortisone
78. Unii-x4w7zr7023
79. .delta.1-6.alpha.-methylhydrocortisone
80. Ncgc00016330-01
81. Prestwick_622
82. Medrol (tn)
83. Pregna-1,4-diene-3,20-dione, 11,17,21-trihydroxy-6-methyl-, (6alpha,11beta)-
84. 6-methyl-prednisolone
85. Methylprednisolone [usp:inn:ban:jan]
86. Opera_id_1576
87. Prestwick0_000279
88. Prestwick1_000279
89. Prestwick2_000279
90. Prestwick3_000279
91. 6alpha-methyl Prednisolone
92. 11-beta,17,21-trihydroxy-6-alpha-methylpregna-1,4-diene-3,20-dione
93. 6alpha-methyl-11beta,17alpha,21-trihydroxy-1,4-pregnadiene-3,20-dione
94. Pregna-1,4-diene-3,20-dione, 11beta,17,21-trihydroxy-6alpha-methyl-
95. Chembl650
96. Ec 201-476-4
97. 6.alpha.-methylprednisolone
98. Schembl5084
99. Bspbio_000158
100. 4-08-00-03498 (beilstein Handbook Reference)
101. Mls001148159
102. Mls002207191
103. Spbio_002377
104. Methylprednisolone [mi]
105. Bpbio1_000174
106. Gtpl7088
107. Methylprednisolone [inn]
108. Methylprednisolone [jan]
109. Dtxsid7023300
110. Methylprednisolone [hsdb]
111. Methylprednisolone [vandf]
112. Hms1568h20
113. Hms2090b13
114. Hms2095h20
115. Hms2230d16
116. Hms3259j04
117. Hms3712h20
118. Methylprednisolone [mart.]
119. 6alpha-methylprednisolone, >=98%
120. Methylprednisolone [usp-rs]
121. Methylprednisolone [who-dd]
122. Hy-b0260
123. Nsc19987
124. Zinc3875560
125. Tox21_110376
126. Tox21_302018
127. Bdbm50103616
128. Lmst02030178
129. Methylprednisolone (jp17/usp/inn)
130. S1733
131. Akos015969744
132. Pregna-1,4-diene-3,20-dione, 6-alpha-methyl-11-beta-17,21-trihydroxy-
133. Tox21_110376_1
134. Ccg-220279
135. Db00959
136. Ks-1273
137. Methylprednisolone [green Book]
138. Nc00691
139. Methylprednisolone [orange Book]
140. Methylprednisolone [ep Monograph]
141. Ncgc00022735-03
142. Ncgc00022735-06
143. Ncgc00255269-01
144. Methylprednisolone [usp Monograph]
145. Nci60_001657
146. M1665
147. Neo-medrol Component Methylprednisolone
148. C16437
149. D00407
150. Methylprednisolone Component Of Neo-medrol
151. 010m591
152. A855290
153. Q417222
154. Sr-01000003089
155. Q-201395
156. Sr-01000003089-2
157. 6-alpha-methylprednisolone 100 Microg/ml In Methanol
158. Brd-k35240538-001-03-1
159. Brd-k35240538-001-11-4
160. Brd-k35240538-001-26-2
161. Methylprednisolone Acetate Impurity B [ep Impurity]
162. 6alpha-methylprednisolone, Vetranal(tm), Analytical Standard
163. 1,4-pregnadien-6alpha-methyl-11beta, 17, 21-triol-3, 20-dione
164. 11?,17?,21-trihydroxy-6?-methyl-1,4-pregnadiene-3,20-dione
165. 11beta,17,21-trihydroxy-6alpha-methyl-1,4-pregnadiene-3,20-dione
166. Methylprednisolone, British Pharmacopoeia (bp) Reference Standard
167. Methylprednisolone, European Pharmacopoeia (ep) Reference Standard
168. Pregna-1,20-dione, 11.beta.,17,21-trihydroxy-6.alpha.-methyl-
169. Pregna-1,20-dione, 6.alpha.-methyl-11.beta.-17,21-trihydroxy-
170. (6?,11?)-11,17,21-trihydroxy-6-methylpregna-1,4-diene-3,20-dione
171. (6a,11b)-11,17,21-trihydroxy-6-methylpregna-1,4-diene-3,20-dione
172. 11beta,17alpha,21-trihydroxy-6alpha-methylpregna-1,4-diene-3,20-dione
173. Methylprednisolone Hydrogen Succinate Impurity A [ep Impurity]
174. Methylprednisolone, United States Pharmacopeia (usp) Reference Standard
175. 11.beta.,17,21-trihydroxy-6.alpha.-methylpregna-1,4-diene-3,20-dione
176. Methylprednisolone For System Suitability A, Europepharmacopoeia (ep) Reference Standard
177. Methylprednisolone For System Suitability, European Pharmacopoeia (ep) Reference Standard
178. Methylprednisolone, Pharmaceutical Secondary Standard; Certified Reference Material
179. Pregna-1,20-dione, 11,17,21-trihydroxy-6-methyl-, (6.alpha.,11.beta.)-
180. Pregna-1,4-diene-3,20-dione, 11,17,21-trihydroxy-6-methyl-, (6i+/-,11i(2))-
| Molecular Weight | 374.5 g/mol |
|---|---|
| Molecular Formula | C22H30O5 |
| XLogP3 | 1.9 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 2 |
| Exact Mass | 374.20932405 g/mol |
| Monoisotopic Mass | 374.20932405 g/mol |
| Topological Polar Surface Area | 94.8 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 754 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 8 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 8 | |
|---|---|
| Drug Name | A-methapred |
| PubMed Health | Methylprednisolone |
| Drug Classes | Endocrine-Metabolic Agent, Immune Suppressant |
| Active Ingredient | Methylprednisolone sodium succinate |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 125mg base/vial; eq 40mg base/vial |
| Market Status | Prescription |
| Company | Hospira |
| 2 of 8 | |
|---|---|
| Drug Name | Medrol |
| PubMed Health | Methylprednisolone |
| Drug Classes | Endocrine-Metabolic Agent, Immune Suppressant |
| Drug Label | MEDROL Tablets contain methylprednisolone which is a glucocorticoid. Glucocorticoids are adrenocortical steroids, both naturally occurring and synthetic, which are readily absorbed from the gastrointestinal tract. Methylprednisolone occurs as a white... |
| Active Ingredient | Methylprednisolone |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 32mg; 8mg; 4mg; 2mg; 16mg |
| Market Status | Prescription |
| Company | Pharmacia And Upjohn |
| 3 of 8 | |
|---|---|
| Drug Name | Methylprednisolone |
| Drug Label | Methylprednisolone Tablets contain methylprednisolone which is a glucocorticoid. Glucocorticoids are adrenocortical steroids, both naturally occurring and synthetic, which are readily absorbed from the gastrointestinal tract. Methylprednisolone occur... |
| Active Ingredient | Methylprednisolone |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 32mg; 8mg; 4mg; 16mg |
| Market Status | Prescription |
| Company | Vintage Pharms; Jubilant Cadista; Sandoz; Duramed Pharms Barr; Watson Labs |
| 4 of 8 | |
|---|---|
| Drug Name | Solu-medrol |
| Active Ingredient | Methylprednisolone sodium succinate |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 2gm base/vial; eq 40mg base/vial; eq 125mg base/vial; eq 500mg base/vial; eq 1gm base/vial |
| Market Status | Prescription |
| Company | Pharmacia And Upjohn |
| 5 of 8 | |
|---|---|
| Drug Name | A-methapred |
| PubMed Health | Methylprednisolone |
| Drug Classes | Endocrine-Metabolic Agent, Immune Suppressant |
| Active Ingredient | Methylprednisolone sodium succinate |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 125mg base/vial; eq 40mg base/vial |
| Market Status | Prescription |
| Company | Hospira |
| 6 of 8 | |
|---|---|
| Drug Name | Medrol |
| PubMed Health | Methylprednisolone |
| Drug Classes | Endocrine-Metabolic Agent, Immune Suppressant |
| Drug Label | MEDROL Tablets contain methylprednisolone which is a glucocorticoid. Glucocorticoids are adrenocortical steroids, both naturally occurring and synthetic, which are readily absorbed from the gastrointestinal tract. Methylprednisolone occurs as a white... |
| Active Ingredient | Methylprednisolone |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 32mg; 8mg; 4mg; 2mg; 16mg |
| Market Status | Prescription |
| Company | Pharmacia And Upjohn |
| 7 of 8 | |
|---|---|
| Drug Name | Methylprednisolone |
| Drug Label | Methylprednisolone Tablets contain methylprednisolone which is a glucocorticoid. Glucocorticoids are adrenocortical steroids, both naturally occurring and synthetic, which are readily absorbed from the gastrointestinal tract. Methylprednisolone occur... |
| Active Ingredient | Methylprednisolone |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 32mg; 8mg; 4mg; 16mg |
| Market Status | Prescription |
| Company | Vintage Pharms; Jubilant Cadista; Sandoz; Duramed Pharms Barr; Watson Labs |
| 8 of 8 | |
|---|---|
| Drug Name | Solu-medrol |
| Active Ingredient | Methylprednisolone sodium succinate |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 2gm base/vial; eq 40mg base/vial; eq 125mg base/vial; eq 500mg base/vial; eq 1gm base/vial |
| Market Status | Prescription |
| Company | Pharmacia And Upjohn |
Anti-Inflammatory Agents, Steroidal; Antiemetics; Glucocorticoids, Synthetic; Glucocorticoids, Topical; Neuroprotective Agents
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
MEDICATION (VET): Treatment with methylprednisolone may be helpful if instituted within the first few hours of /spinal cord/ injury.
Kahn, C.M. (Ed.); The Merck Veterinary Manual 9th ed. Merck & Co. Whitehouse Station, NJ. 2005, p. 1024
MEDICATION (VET): Glucocorticoids are usually contraindicated in animals with meningitis or meningoencephalitis with an infectious etiology; however, a high-dose, short-term course of ... methylprednisolone may control life-threatening complications such as acute cerebral edema and impending brain herniation.
Kahn, C.M. (Ed.); The Merck Veterinary Manual 9th ed. Merck & Co. Whitehouse Station, NJ. 2005, p. 1047
MEDICATION (VET): In cats with mild to moderate inflammatory bowel disease (IBD) or relapse of clinical signs, and in those in which administration of oral medication is difficult, methylprednisolone ... may be effective as the sole treatment or as an adjunct to prednisone and metronidazole.
Kahn, C.M. (Ed.); The Merck Veterinary Manual 9th ed. Merck & Co. Whitehouse Station, NJ. 2005, p. 339
For more Therapeutic Uses (Complete) data for METHYLPREDNISOLONE (29 total), please visit the HSDB record page.
/SRP: High dose/ /Methylprednisolone, when administered as a therapeutic agent, has been /associated with hallucinations. /From table/
Ellenhorn, M.J. and D.G. Barceloux. Medical Toxicology - Diagnosis and Treatment of Human Poisoning. New York, NY: Elsevier Science Publishing Co., Inc. 1988., p. 666
Contraindicted in patients with systemic fungal infections. Adverse reactions include sodium and fluid retention, potassium depletion, muscle weakness, osteoporosis, peptic ulcer, thin fragile skin, development of Cushingoid state, glaucoma, cataracts, and negative nitrogen balance. May mask signs of infection and new infections may appear during use. May increase requirements for hypoglycemic agents in diabetic patients.
Hussar, D.A. (ed.). Modell's Drugs in Current Use and New Drugs. 38th ed. New York, NY: Springer Publishing Co., 1992., p. 104
We describe a 61 year-old caucasian male diagnosed with rheumatoid arthritis. He was started on methylprednisolone pulses because of a severe flare of symmetric polyarthritis while he was on weekly intramuscular methotrexate and low-dose oral prednisone. After the second pulse of methylprednisolone the patient suddenly developed severe abdominal pain with free air under the right hemidiaphragm in the chest roentgenogram. The emergency surgery revealed the perforation of a colonic diverticulum. We suggest that methylprednisolone pulses should be carefully used in those patients over 50 years of age and/or people with demonstrated or suspected diverticular disease.
PMID:9572644 Candelas G et al; Scand J Rheumatol 27 (2): 152-3 (1998)
The immunosuppressive effects of glucocorticoids may result in activation of latent infection or exacerbation of intercurrent infections, including those caused by Candida, Mycobacterium, Toxoplasma, Strongyloides, Pneumocystis, Cryptococcus, Nocardia, or Ameba. Glucocorticoids should be used with great care in patients with known or suspected Strongyloides (threadworm) infection. In such patients, glucocorticoid-induced immunosuppression may lead to Strongyloides hyperinfection and dissemination with widespread larval migration, often accompanied by severe enterocolitis and potentially fatal gram-negative septicemia. /Corticosteroids/
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 3033
For more Drug Warnings (Complete) data for METHYLPREDNISOLONE (33 total), please visit the HSDB record page.
Oral and intramuscular methylprednisolone are indicated for a number of endocrine, rheumatic, collagen, dermatologic, allergic, ophthalmic, respiratory, hematologic, neoplastic, edematous, gastrointestinal, nervous system, and other disorders. Intra-articular and soft tissue injections are indicated for short term treatment of acute gouty arthritis, acute and subactute bursitis, acute nonspecific tenosynovitis, epicondylitis, rheumatoid arthritis, and synovitis of osteoarthritis. Intralesional injections are indicated for alopecia areata, discoid lupus erythematosus, keloids, lichen planus, lichen simplex chronicus and psoriatic plaques, necrobiosis lipoidica diabeticorum, and localized hypertrophic infiltrated inflammatory lesions of granuloma annulare.
FDA Label
Corticosteroids bind to the glucocorticoid receptor, inhibiting pro-inflammatory signals, and promoting anti-inflammatory signals. Corticosteroids have a wide therapeutic window as patients may require doses that are multiples of what the body naturally produces. Patients taking corticosteroids should be counselled regarding the risk of hypothalamic-pituitary-adrenal axis suppression and increased susceptibility to infections.
Anti-Inflammatory Agents
Substances that reduce or suppress INFLAMMATION. (See all compounds classified as Anti-Inflammatory Agents.)
Neuroprotective Agents
Drugs intended to prevent damage to the brain or spinal cord from ischemia, stroke, convulsions, or trauma. Some must be administered before the event, but others may be effective for some time after. They act by a variety of mechanisms, but often directly or indirectly minimize the damage produced by endogenous excitatory amino acids. (See all compounds classified as Neuroprotective Agents.)
Glucocorticoids
A group of CORTICOSTEROIDS that affect carbohydrate metabolism (GLUCONEOGENESIS, liver glycogen deposition, elevation of BLOOD SUGAR), inhibit ADRENOCORTICOTROPIC HORMONE secretion, and possess pronounced anti-inflammatory activity. They also play a role in fat and protein metabolism, maintenance of arterial blood pressure, alteration of the connective tissue response to injury, reduction in the number of circulating lymphocytes, and functioning of the central nervous system. (See all compounds classified as Glucocorticoids.)
Antiemetics
Drugs used to prevent NAUSEA or VOMITING. (See all compounds classified as Antiemetics.)
H02AB04
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
D - Dermatologicals
D07 - Corticosteroids, dermatological preparations
D07A - Corticosteroids, plain
D07AA - Corticosteroids, weak (group i)
D07AA01 - Methylprednisolone
D - Dermatologicals
D10 - Anti-acne preparations
D10A - Anti-acne preparations for topical use
D10AA - Corticosteroids, combinations for treatment of acne
D10AA02 - Methylprednisolone
H - Systemic hormonal preparations, excl. sex hormones and insulins
H02 - Corticosteroids for systemic use
H02A - Corticosteroids for systemic use, plain
H02AB - Glucocorticoids
H02AB04 - Methylprednisolone
Absorption
Oral methylprednisolone has 89.9% the bioavailability of oral methylprednisolone acetate, while rectal methylprednisolone has 14.2% the bioavailability. Intravitreal methylprednisolone has a Tmax of 2.5h. Approximately 1/10 of an oral or IV dose of methylprednisolone will reach the vitreous humor. Further data regarding the absorption of methylprednisolone are not readily available.
Route of Elimination
Methylprednisolone and its metabolites have been collected in urine in humans. A study in dogs showed 25-31% elimination in urine and 44-52% elimination in feces.
Volume of Distribution
The average volume of distribution of methylprednisolone is 1.38L/kg.
Clearance
The average plasma clearance of methylprednisolone is 336mL/h/kg.
ORAL ABSORPTION IN SINGLE-DOSE STUDY OF 12 NORMAL MALE VOLUNTEERS. MEAN BIOAVAIL AFTER ORAL ADMIN 89.9%, INDICATING BETTER SYSTEMIC AVAIL OF ESTER THAN ALC. AVG ELIMINATION RATE CONSTANT AFTER ORAL ADMIN OF ESTER & ALC 0.290 H-1, HALF-LIFE OF 2.39 HR.
PMID:455892 GARG DC ET AL; CLIN PHARMACOL THER 26 (2): 232-9 (1979)
The pharmacokinetics of methylprednisolone (MP) were studied in five normal subjects following intravenous doses of 20, 40 and 80 mg methylprednisolone sodium succinate (MPSS) and an oral dose of 20 mg methylprednisolone as 4 x 5 mg tablets. Plasma concentrations of MP and MPSS were measured by both high performance thin layer (h.p.t.l.c.) and high pressure liquid chromatography (h.p.l.c.). 2. The mean values (+/- s.d.) of half-life, mean residence time (MRT), systemic clearance (CL) and volume of distribution at steady state (Vss) of MP following intravenous administration were 1.93 +/- 0.35 h, 3.50 +/- 1.01 h, 0.45 +/- 0.12 lh-1 kg-1 and 1.5 +/- 0.63 1 kg-1, respectively. There was no evidence of dose-related changes in these values. The plasma MP concentration-time curves were superimposable when normalized for dose. 3. The bioavailability of methylprednisolone from the 20 mg tablet was 0.82 +/- 0.11 (s.d.). 4. In vivo hydrolysis of MPSS was rapid with a half-life of 4.14 +/- 1.62 (s.d.) min, and was independent of dose. In contrast, in vitro hydrolysis in plasma, whole blood and red blood cells was slow; the process continuing for more than 7 days. Sodium fluoride did not prevent the hydrolysis of MPSS.
PMID:2655680 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1379824 Al-Habet SM, Rogers HJ; Br J Clin Pharmacol 27 (3): 285-90 (1989)
High-dose methylprednisolone is used to treat acute spinal cord injury (ASCI). The objective of the present study was to determine the pharmacokinetics of the pro-drug methylprednisolone hemisuccinate and methylprednisolone in accident victims with ASCI. The patients (n = 26) were treated with a bolus intravenous loading dose of 30 mg/kg MPHS within 2 hr after injury and this was followed by a maintenance infusion of 5.4 mg/kg/h up to 24 hr. Blood, CSF and saliva samples were collected up to 48 hr after the initial dose and the samples were analyzed by HPLC. Concentration-time data of MPHS and methylprednisolone were analyzed using population pharmacokinetic analysis with NONMEM software. RESULTS: Methylprednisolone hemisuccinate and methylprednisolone could be monitored in plasma and CSF. Methylprednisolone but not methylprednisolone hemisuccinate was present in saliva. High variability was seen in the methylprednisolone hemisuccinate levels in CSF. The pharmacokinetics of the pro-drug and the metabolite were adequately described by a 2-compartment model with exponential distribution models assigned to the interindividual and the residual variability. At steady state, the average measured methylprednisolone concentration in plasma was 12.3+/-7.0 microg/ml and 1.74+/-0.85 microg/ml in CSF. The CSF levels of methylprednisolone could be modeled as a part of the peripheral compartment. This study demonstrated that CSF concentrations of methylprednisolone were sufficiently high after IV. administration and reflected the concentrations of unbound drug in plasma. Salivary levels of methylprednisolone were about 32% of the plasma level and may serve as an easily accessible body fluid for drug level monitoring.
PMID:15487809 Barth J et al; Int J Clin Pharmacol Ther 42 (9): 504-11 (2004)
Sodium fluoride (6--8 mg/ml) inhibits hydrolysis of methylprednisolone acetate to methylprednisolone. An HPLC method for simultaneous determination of hydrocortisone, methylprednisolone and methylprednisolone acetate in plasma is presented. Analysis of plasma samples (containing NaF) for methylprednisolone acetate shows no significant change in concentration over extended periods of storage at -20 degrees C. In vitro hydrolysis of methylprednisolone acetate at 37 degrees C in human whole blood is rapid (average t1/2 = 19 min). In one cat, the bioavailabilities of methylprednisolone acetate rectally was 13% and of methylprednisolone (alcohol) rectally was 26%, relative to intravenous administration of methylprednisolone. In the same cat, the bioavailabilities of methylprednisolone acetate orally was 93% and of methylprednisolone was 82%, relative to intravenous administration of methylprednisolone. All samples collected after oral administration of methylprednisolone acetate to a human subject were found to contain only methylprednisolone (alcohol) indicating hydrolysis of the drug during absorption through the gastrointestinal membrane and/or in the liver. If the ester had the same half-life in blood in vivo as measured in vitro, it would have been measurable in plasma.
PMID:725320 Garg DC et al; Res Commun Chem Pathol Pharmacol 22 (1): 37-48 (1978)
For more Absorption, Distribution and Excretion (Complete) data for METHYLPREDNISOLONE (7 total), please visit the HSDB record page.
The metabolism of methylprednisolone is thought to be mostly mediated by 11beta-hydroxysteroid dehydrogenases and 20-ketosteroid reductases.
METAB OF 6ALPHA-METHYLPREDNISOLONE NA SUCCINATE IN RATS; METABOLITES; 6ALPHA-METHYLPREDNISOLONE, 6ALPHA-METHYL-11BETA,17ALPHA,20BETA- TRIHYDROXY-1,4-PREGNADIEN-3-ONE,21-OIC ACID, & 6ALPHA-METHYL-11BETA,17ALPHA,20,21-TETRAHYDROXY-1,4-PREGNADIEN-3-ONE 21-SUCCINATE.
KITAGAWA H ET AL; OYO YAKURI 13 (2): 249-57 (1977)
Prednisone, prednisolone, and methylprednisolone are currently administered in association with cyclosporin A in the postoperative treatment of transplant patients. The aim of this work was to evaluate the effects of these corticosteroids on the expression of several forms of cytochromes p450, including p450 1A2, 2D6, 2E1, and 3A, and on cyclosporin A oxidase activity in human liver. For this purpose, human hepatocytes prepared from lobectomies were maintained in culture in a serum-free medium, in collagen-coated dishes, for 96-144 hr, in the absence or presence of 50-100 uM corticosteroids, rifampicin, or dexamethasone. To mimic more closely the current clinical protocol, hepatocyte cultures were also co-treated with corticosteroids and cyclosporin A or ketoconazole (a selective inhibitor of cytochromes p450 3A). Cyclosporin A oxidase activity, intracellular retention of cyclosporin A oxidized metabolites within hepatocytes, accumulation of cytochromes p450 proteins and corresponding messages, and de novo synthesis and half-lives of these cytochromes p450 were measured in parallel in these cultures. Our results, obtained from seven different hepatocyte cultures, showed that 1) dexamethasone and prednisone, but not prednisolone or methylprednisolone, were inducers of cytochrome p450 3A, at the level of protein and mRNA accumulation, as well as of cyclosporin A oxidase activity, known to be predominantly catalyzed by these cytochromes p450; 2) although corticosteroids are known to be metabolized in human liver, notably by cytochrome p450 3A, partial or total inhibition of this cytochromes p450 by cyclosporin or ketoconazole, respectively, did not affect the inducing efficiency of these molecules; 3) corticosteroids did not affect the half-life of cytochrome p450 3A or the accumulation of other forms of cytochromes p450, including 1A2, 2D6, and 2E1; 4) chronic treatment of cells with cyclosporin did not affect cytochrome p450 3A accumulation; 5) corticosteroids were all competitive inhibitors of cyclosporin A oxidase in human liver microsomes, with Ki values of 61 + or - 12, 125 + or - 25, 190 + or - 38, and 210 + or - 42 uM for dexamethasone, prednisolone, prednisone, and methylprednisolone, respectively; and 6) chronic treatment of cells with corticosteroids did not influence the excretion of oxidized metabolites of cyclosporin from the cells.
PMID:1614409 Pichard L et al; Mol Pharmacol 41 (6): 1047-55 (1992)
Methylprednisolone has a half life of 2.3h.
IV HALF-LIFE IS APPROX 80 MIN IN DOGS.
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 359
PLASMA HALF-LIFE IS 3-4 HR.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 895
SINGLE DOSE STUDY OF 12 NORMAL MALE VOLUNTEERS. HALF-LIFE OF 2.39 HR.
GARG DC ET AL; CLIN PHARMACOL THER 26(2) 232-9 (1979)
The short term effects of corticosteroids are decreased vasodilation and permeability of capillaries, as well as decreased leukocyte migration to sites of inflammation. Corticosteroids binding to the glucocorticoid receptor mediates changes in gene expression that lead to multiple downstream effects over hours to days. Glucocorticoids inhibit neutrophil apoptosis and demargination; they inhibit phospholipase A2, which decreases the formation of arachidonic acid derivatives; they inhibit NF-Kappa B and other inflammatory transcription factors; they promote anti-inflammatory genes like interleukin-10. Lower doses of corticosteroids provide an anti-inflammatory effect, while higher doses are immunosuppressive. High doses of glucocorticoids for an extended period bind to the mineralocorticoid receptor, raising sodium levels and decreasing potassium levels.
Glucocorticoids are capable of suppressing the inflammatory process through numerous pathways. They interact with specific intracellular receptor proteins in target tissues to alter the expression of corticosteroid-responsive genes. Glucocorticoid-specific receptors in the cell cytoplasm bind with steroid ligands to form hormone-receptor complexes that eventually translocate to the cell nucleus. There these complexes bind to specific DNA sequences and alter their expression. The complexes may induce the transcription of mRNA leading to synthesis of new proteins. Such proteins include lipocortin, a protein known to inhibit PLA2a and thereby block the synthesis of prostaglandins, leukotrienes, and PAF. Glucocorticoids also inhibit the production of other mediators including AA metabolites such as COX, cytokines, the interleukins, adhesion molecules, and enzymes such as collagenase. /Glucocorticoids/
Kahn, C.M. (Ed.); The Merck Veterinary Manual 9th ed. Merck & Co. Whitehouse Station, NJ. 2005, p. 2128








