



API Suppliers

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0

USA (Orange Book)
0

Europe
0

Canada

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
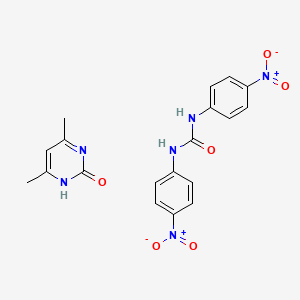

1. Nicarbazine
1. 330-95-0
2. Nicarbazine
3. Nicoxin
4. Nicrazin
5. Nirazin
6. Nicrazine
7. Nicarb
8. Mk 75
9. Ai3-60130
10. Urea, N,n'-bis(4-nitrophenyl)-, Compd. With 4,6-dimethyl-2(1h)-pyrimidinone (1:1)
11. 11p9nua12u
12. 1,3-bis(4-nitrophenyl)urea,4,6-dimethyl-1h-pyrimidin-2-one
13. Chebi:81725
14. Nsc-7171
15. Nicarbasin
16. 1,3-bis(4-nitrophenyl)urea 4,6-dimethylpyrimidin-2-ol (1:1)
17. Nicarbazin [ban]
18. Mk 75 (van)
19. Hsdb 7466
20. Nsc 7171
21. Einecs 206-359-1
22. 1,3-bis(4-nitrophenyl)urea;4,6-dimethylpyrimidin-2-ol
23. Unii-11p9nua12u
24. 4,4'-dinitrocarbanilide, 4,6-dimethyl-2-pyrimidinol
25. Nicarbazin [mi]
26. 1,3-bis(4-nitrophenyl)urea; 4,6-dimethyl-1h-pyrimidin-2-one
27. Nicarbazin [hsdb]
28. Nicarbazin [mart.]
29. Dsstox_cid_14762
30. Dsstox_gsid_34762
31. Chembl95855
32. Schembl578675
33. Nicarbazin [green Book]
34. 1,3-bis(4-nitrophenyl)urea-4,6-dimethylpyrimidin-2-ol
35. Dtxsid6034762
36. 4, 4,6-dimethyl-2-pyrimidinol
37. Nsc7171
38. 1,3-bis(4-nitrophenyl)urea;4,6-dimethyl-1h-pyrimidin-2-one
39. Tox21_304000
40. S4866
41. Akos015900412
42. Akos025310650
43. Carbanilide, 4,4'-dinitro-, Compd. With 4,6-dimethyl-2-pyrimidinol (1:1)
44. Ccg-268958
45. Urea, N,n-bis(4-nitrophenyl)-, Compound With 4,6-dimethyl-2(1h)-pyrimidinone (1:1)
46. Ncgc00357219-01
47. As-13365
48. Cas-330-95-0
49. Nicarbazine 100 Microg/ml In Acetonitrile
50. Hy-107814
51. Cs-0030690
52. Ft-0603380
53. C18389
54. D95916
55. Nicarbazin, Vetranal(tm), Analytical Standard
56. 330n950
57. A821619
58. Nicarbazin, Antibiotic For Culture Media Use Only
59. Q2020694
60. W-106788
61. 1,3-bis(4-nitrophenyl)urea; 4,6-dimethylpyrimidin-2-ol
62. 1,3-bis(4-nitrophenyl)urea-4,6-dimethylpyrimidin-2-ol (1:1)
63. 1,3-bis-(4-nitro-phenyl)-urea And 4,6-dimethyl-pyrimidin-2-ol
64. 1,3-bis(4-nitrophenyl)urea Complex With 4,6-dimethylpyrimidin-2-ol
65. 1,3-bis(4-nitrophenyl)urea Compound With 4,6-dimethylpyrimidin-2-ol (1:1)
66. Carbanilide,4'-dinitro-, Compd. With 4,6-dimethyl-2-pyrimidinol (1:1)
67. Urea,n'-bis(4-nitrophenyl)-, Compd. With 4,6-dimethyl-2(1h)-pyrimidinone (1:1)
| Molecular Weight | 426.4 g/mol |
|---|---|
| Molecular Formula | C19H18N6O6 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 2 |
| Exact Mass | 426.12878232 g/mol |
| Monoisotopic Mass | 426.12878232 g/mol |
| Topological Polar Surface Area | 174 Ų |
| Heavy Atom Count | 31 |
| Formal Charge | 0 |
| Complexity | 582 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
/VET:/ Nicarbazin has been used in starter rations for several decades as an aid in the prevention of fecal and intestinal coccidiosis in broiler chickens. It may be used in combination with ionophore coccidiostatics.
Joint FAO/WHO Expert Committee on Food Additives; WHO Food Additive Series 41: Toxicological Evaluation of Ceratain Veterinary Drug Residues in Food: Nicarbazin (1998). Available from, as of November 11, 2006: https://www.inchem.org/documents/jecfa/jecmono/v041je10.htm
/VET:/This coccidiostat /nicarbazin/ is used in broilers.
Kahn, C.M. (Ed.); The Merck Veterinary Manual 9th ed. Merck & Co. Whitehouse Station, NJ. 2005, p. 2260
/VET:/ It should not be fed to layers, as it can cause discoloration and reduced hatchability of eggs (although the effect is reversible once the nicarbazin is withdrawn). It also may result in reduced heat tolerance in birds exposed to high temperature and humidity;
Kahn, C.M. (Ed.); The Merck Veterinary Manual 9th ed. Merck & Co. Whitehouse Station, NJ. 2005, p. 2260
Coccidiostats
Agents useful in the treatment or prevention of COCCIDIOSIS in man or animals. (See all compounds classified as Coccidiostats.)
Rats received single oral doses of 1, 5, or 10 mg/kg bw nicarbazin; one animal at each dose was killed 6 or 18 hr after treatment, and the blood concentrations of the phenylurea and pyrimidone components were determined. Low concentrations of the phenylurea component were detected at 6 hr, but it was not detected at 18 hr. The pyrimidone component was found at considerably higher concentrations, which increased between 6 and 18 hr. Qualitatively similar findings were obtained in rats given oral doses of 0.1, 1, or 5 mg/kg bw per day for eight days and killed 4 or 24 hr after the last dose. The blood concentrations of the pyrimidone component were dose-related, while those of the phenylurea component showed a flat response. In the latter experiment, urine collected for 5 hr after the last dose contained dose-related concentrations of each component of nicarbazin, although the concentrations of the pyrimidone were an order of magnitude higher than those of the phenylurea component
USEPA/Office of Pesticide Programs; Pesticide Fact Sheet for Nicarbazin (November 2005). Available from, as of November 2, 2006: https://www.epa.gov/opprd001/factsheets/
...Nicarbazin, in the form of 4,4'-dinitrocarbanilide (DNC), was detected in 39 of the 190 eggs analysed. ... The concentrations of both the DNC and 4,6-dimethyl-2-hydroxypyrimidine (DHP) components of the drug in eggs were proportional to feed levels. The maximum feed nicarbazin concentration of 12.1 mg/kg (8.6 mg/kg DNC and 3.5 mg/kg DHP) gave rise to mean maximum whole egg concentrations of 631 micrograms/kg DNC and 51.8 micrograms/kg DHP. After withdrawal of the experimental diet, DNC was undetectable in eggs after 12 days and DHP after 3 days. Feed contaminated with nicarbazin at concentrations greater than about 2 mg/kg gave rise to egg DNC residues at concentrations greater than the Differential Action Limit (DAL) set by the UK (100 micrograms/kg). DNC was contained almost entirely in the yolk of the egg, whereas DHP was distributed between albumen and yolk in a ratio of approximately 3:1.
PMID:11103266 Cannavan A et al; Food Addit Contam 17 (10): 829-36 (2000)
Nicarbazin interferes with the formation of the vitelline membrane, separating the egg yolk and egg white. The exact mode of action is unknown, although it is thought nicarbazin interferes with cholesterol metabolism in the formation of the membrane. Eggs from treated birds are described as mottled in appearance, reflecting a porous vitelline membrane. The effect on hatchability is a function of time and dose and the effect is reversible.
USEPA/Office of Pesticide Programs; Pesticide Fact Sheet for Nicarbazin (November 2005). Available from, as of November 2, 2006: https://www.epa.gov/opprd001/factsheets/


