



API Suppliers

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
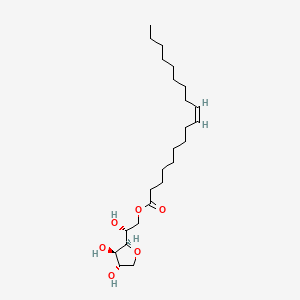

1. Sorbitan Oleate
2. Span 80
1. Sorbitan, Mono-(9z)-9-octadecenoate
2. Arlacel 80
3. Span 80
4. 1338-43-8
5. Anhydrosorbitol Monooleate
6. Sorbitan Mono-oletae
7. Sorbitan Oleate
8. Sorbitan, Mono-9-octadecenoate
9. 06xea2vd56
10. Ins No.494
11. Ins-494
12. 1,4-anhydro-6-o-[(9z)-octadec-9-enoyl]-d-glucitol
13. Nsc-406239
14. Ncgc00164240-01
15. Sorbitan Oleate [inn]
16. E-494
17. Dsstox_cid_7397
18. Dsstox_rid_78437
19. D-glucitol, 1,4-anhydro-, 6-(9-octadecenoate)
20. Dsstox_gsid_27397
21. Sorbitan Mono-oleate
22. Cas-1338-43-8
23. Sorbitan Monooleate [usan]
24. Sorbitani Oleas
25. Glycomul O
26. Sorbitan O
27. Alkamuls Smo
28. Armotan Mo
29. Dehymuls Smo
30. Lonzest Smo
31. Nsc 406239
32. Oleate De Sorbitan
33. Kosteran O 1
34. Oleato De Sorbitano
35. Crill 4
36. Sorbester P 17
37. Disponil 100
38. Montan 80
39. Newcol 80
40. Nonion Op80r
41. Radiasurf 7155
42. Sorgen 40
43. Sorgen 40a
44. Montane 80 Vga
45. Rheodol Ao 10
46. Atmer 05
47. Emasol O 10
48. Emasol O 10f
49. Kemmat S 80
50. Nikkol So 10
51. Nikkol So-15
52. Sorbon S 80
53. Emasol 410
54. Rheodol Sp-o 10
55. Rikemal O 250
56. Sorbitan, Mono-9-octadecenoate, (z)-
57. Emsorb 2500
58. Ionet S-80
59. Span-80
60. S 271 (surfactant)
61. Nissan Nonion Op 80r
62. Mannide Monooleate, Liquid
63. Dianhydromannitol Monooleate
64. Schembl4504
65. Span(r) 80, For Gc
66. Unii-06xea2vd56
67. Monodehydrosorbitol Monooleate
68. Sorbitan Monooleic Acid Ester
69. Ccris 710
70. Sorbitani Oleas [inn-latin]
71. Mannide Monooleate, From Plant
72. Sorbitan Esters Sorbitan Oleate
73. Sorbitan Oleate [inci]
74. Ml 55f
75. Mo 33f
76. S-max 80
77. Sorbitan Monooleate [usan:nf]
78. Chembl1894187
79. Dtxsid6027397
80. Sorbitan Monooleate [ii]
81. Sorbitan Oleate [mart.]
82. Hsdb 5822
83. Oleate De Sorbitan [inn-french]
84. Sorbitan Monooleate [fcc]
85. Span #80 (sorbitan Monooleate)
86. Chebi:183688
87. Span(r) 80, Nonionic Surfactant
88. Sorbitan Monooleate [hsdb]
89. Oleato De Sorbitano [inn-spanish]
90. Sorbitan Monooleate [vandf]
91. Zinc8214458
92. Einecs 215-665-4
93. Tox21_113539
94. Tox21_300389
95. Mfcd00080948
96. Sorbitan Monooleate, Saj First Grade
97. Akos024431316
98. Sorbitan Mono-oleate [who-dd]
99. Sorbitan Oleate [ep Monograph]
100. Ncgc00164240-02
101. Ncgc00254386-01
102. S 80
103. Sorbitan Esters, Mono(z)-9-octadecenoate
104. G 946
105. O 250
106. S 270
107. S0060
108. Sorbitan Esters Sorbitan Oleate [mi]
109. 1,4-anhydro-d-glucitol, 6-(9-octadecenoate)
110. J-006443
111. Q2303156
112. 1,4-anhydro-d-glucitol, 6-(9-octadecenoate
113. Span(r) 80, Viscosity 1000-2000 Mpa.s (20 C)
114. [(2r)-2-[(2r,3r,4s)-3,4-dihydroxyoxolan-2-yl]-2-hydroxyethyl] (z)-octadec-9-enoate
115. Sorbitan Monooleate. (compound Usually Contains Also Associated Fatty Acids.)
| Molecular Weight | 428.6 g/mol |
|---|---|
| Molecular Formula | C24H44O6 |
| XLogP3 | 6 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 19 |
| Exact Mass | 428.31378912 g/mol |
| Monoisotopic Mass | 428.31378912 g/mol |
| Topological Polar Surface Area | 96.2 Ų |
| Heavy Atom Count | 30 |
| Formal Charge | 0 |
| Complexity | 453 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
1. 1= PRACTICALLY NONTOXIC: PROBABLE ORAL LETHAL DOSE (HUMAN) ABOVE 15 G/KG; MORE THAN 1 QT (2.2 LB) FOR 70 KG PERSON (150 LB). /SORBITAN MONOSTEARATE/
Gosselin, R.E., H.C. Hodge, R.P. Smith, and M.N. Gleason. Clinical Toxicology of Commercial Products. 4th ed. Baltimore: Williams and Wilkins, 1976., p. II-181
Surface-Active Agents
Agents that modify interfacial tension of water; usually substances that have one lipophilic and one hydrophilic group in the molecule; includes soaps, detergents, emulsifiers, dispersing and wetting agents, and several groups of antiseptics. (See all compounds classified as Surface-Active Agents.)
WHEN DIGESTED, BOTH THE FATTY ACID & THE POLYHYDRIC ALCOHOL SORBITAN ARE ABSORBED, BUT THE LATTER IS COMPLETELY EXCRETED IN URINE. /SORBITAN MONOSTEARATE/
Gosselin, R.E., H.C. Hodge, R.P. Smith, and M.N. Gleason. Clinical Toxicology of Commercial Products. 4th ed. Baltimore: Williams and Wilkins, 1976., p. II-181


