



API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
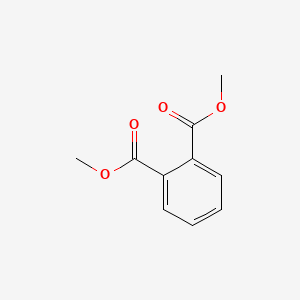

1. Dimethyl Phthalate, Conjugate Diacid
2. Dimethylphthalate
1. 131-11-3
2. Avolin
3. Dimethylphthalate
4. Fermine
5. Solvanom
6. Mipax
7. Palatinol M
8. Solvarone
9. Dimethyl O-phthalate
10. Phthalic Acid Dimethyl Ester
11. Unimoll Dm
12. Repeftal
13. Methyl Phthalate
14. Dimethyl Benzene-1,2-dicarboxylate
15. Dimethyl 1,2-benzenedicarboxylate
16. 1,2-benzenedicarboxylic Acid, Dimethyl Ester
17. Phthalsaeuredimethylester
18. Phthalic Acid, Dimethyl Ester
19. Dimethyl Benzene-o-dicarboxylate
20. Ent 262
21. Dimethylphtalate
22. Dimethyl Benzeneorthodicarboxylate
23. Nsc 15398
24. 1,2-benzenedicarboxylic Acid, 1,2-dimethyl Ester
25. Dmf, Insect Repellent
26. Nsc-15398
27. Benzenedicarboxylic Acid, Dimethyl Ester
28. 08x7f5udjm
29. Chebi:4609
30. Chembl323348
31. Ntm
32. Phthalic Acid, Bis-methyl Ester
33. Ncgc00090692-02
34. Dsstox_cid_2455
35. Dimethyl Phthalate 5000 Microg/ml In Methanol
36. Dsstox_rid_76596
37. Dsstox_gsid_22455
38. Dimethyl Phthalate, >=99%
39. Caswell No. 380
40. Dmf (insect Repellant)
41. Rcra Waste Number U102
42. Cas-131-11-3
43. Dimethyl Phthalate [bsi:iso]
44. Ccris 2674
45. Phtalate De Dimethyle
46. Hsdb 1641
47. Phthalic Acid Dimethyl Ester (d6)
48. Phthalsaeuredimethylester [german]
49. Phtalate De Dimethyle [iso-french]
50. Einecs 205-011-6
51. Dimethyl Phthalate, Pestanal(r), Analytical Standard
52. Rcra Waste No. U102
53. Unii-08x7f5udjm
54. Dimethylester Kyseliny Ftalove [czech]
55. Epa Pesticide Chemical Code 028002
56. Dimethylester Kyseliny Ftalove
57. Ai3-00262
58. Density Standard 1191 Kg/m3
59. Dimethyl-phthalate
60. Kemester Dmp
61. Kodaflex Dmp
62. 1,dimethyl Ester
63. Uniplex 110
64. Dimethyl Orthophthalate
65. 1,2-dimethyl Benzene-1,2-dicarboxylate
66. 1,2-dimethyl Phthalate
67. Dimethyl Phthalate, 99%
68. Dimethyl Phthalate [usp]
69. Phthalic Acid Methyl Ester
70. Ec 205-011-6
71. Wln: 1ovr Bvo1
72. 1,2-benzenedicarboxylic Acid 1,2-dimethyl Ester
73. Schembl34630
74. Dimethyl Phthalate, Ar,99%
75. Dimethyl Phthalate, Cp,99%
76. Mls002177801
77. Bidd:er0349
78. Dimethyl Phthalate [ii]
79. Dimethyl Phthalate [mi]
80. Dimethyl Phthalate [iso]
81. Dtxsid3022455
82. Dimethyl 1,2-benzendicarboxylate
83. Dimethyl Phthalate [hsdb]
84. Dimethyl Phthalate [inci]
85. Zinc391885
86. Dimethyl Phthalate [mart.]
87. Amy40794
88. Dimethyl Phthalate [who-dd]
89. Hy-n7106
90. Nsc15398
91. Tox21_113536
92. Tox21_202145
93. Tox21_301045
94. Bdbm50090983
95. Mfcd00008425
96. S5378
97. Stl283931
98. Akos008969337
99. Ccg-266531
100. Db13336
101. Ncgc00090692-01
102. Ncgc00090692-03
103. Ncgc00090692-04
104. Ncgc00090692-05
105. Ncgc00090692-06
106. Ncgc00254947-01
107. Ncgc00259694-01
108. Bs-20466
109. Smr000777937
110. Db-062803
111. Benzene-1,2-dicarboxylic Acid Dimethyl Ester
112. Cs-0013572
113. Ft-0625095
114. P0302
115. En300-18366
116. Dimethyl Phthalate, Saj Special Grade, >=99.0%
117. Q423551
118. J-005938
119. Z57902306
120. Density Standard 1191 Kg/m3, H&d Fitzgerald Ltd. Quality
121. Phthalic Acid, Bis-methyl Ester 100 Microg/ml In Acetonitrile
122. Phthalic Acid, Bis-methyl Ester 1000 Microg/ml In Acetonitrile
123. Phthalic Acid, Bis-methyl Ester 1000 Microg/ml In N-hexane
124. Benzene,1,2-dicarboxylic Acid,dimethyl Ester (phthalic Acid,dimethyl Ester)
| Molecular Weight | 194.18 g/mol |
|---|---|
| Molecular Formula | C10H10O4 |
| XLogP3 | 1.6 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 4 |
| Exact Mass | 194.05790880 g/mol |
| Monoisotopic Mass | 194.05790880 g/mol |
| Topological Polar Surface Area | 52.6 Ų |
| Heavy Atom Count | 14 |
| Formal Charge | 0 |
| Complexity | 200 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Insect Repellents
Substances causing insects to turn away from them or reject them as food. (See all compounds classified as Insect Repellents.)
Fixatives
Agents employed in the preparation of histologic or pathologic specimens for the purpose of maintaining the existing form and structure of all of the constituent elements. Great numbers of different agents are used; some are also decalcifying and hardening agents. They must quickly kill and coagulate living tissue. (See all compounds classified as Fixatives.)
P - Antiparasitic products, insecticides and repellents
P03 - Ectoparasiticides, incl. scabicides, insecticides and repellents
P03B - Insecticides and repellents
P03BX - Other insecticides and repellents
P03BX02 - Dimethylphthalate
/After/ oral admin of (14)C dimethyl phthalate to rats or mice, radioactivity was found in the blood and various tissues. Maximum values for radioactivity were observed within 1 hr. Tissue radioactivity was highest in the kidneys, followed in decreasing order by the liver, fat, and spleen. After 24 hr, 91% of the admin dose had been excreted in the urine and 4.1% in the feces.
ITC/USEPA; Information Review #214 (Draft) Alkyl Phthalates p.4 (1980)
The percutaneous absorption of a series of phthalate esters, dimethylphthalate, diethylphthalate, dibutyl phthalate, and di-(2-ethylhexyl) phthalate, was measured through human and rat epidermal membranes mounted in glass diffusion cells. The esters were applied directly to the epidermal membranes. Following application to the membranes, a lag phase followed by a linear phase of absorption was detected for each phthalate diester. Human skin was less permeable than rat skin for all four diesters. There appeared to be a trend to an increasing lag time with increasing molecular weight, but this relationship did not always hold true. The phthalate diesters were determined to have a 300 fold range of aqueous solubility and a wide range of lipophilicity. Once the diesters had contacted the human epidermal membrane, a slight increase in the permeability of the skin was detected. Relatively large changes in permeability were detected in the membrane following exposure.
PMID:3691429 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1474516 Scott RC et al; Environ Health Perspect 74: 223-27 (1987)
This study examined the extent of dermal absorption of a series of phthalate diesters in the rat. Those tested were dimethyl, diethyl, dibutyl, diisobutyl, dihexyl, di(2-ethylhexyl), diisodecyl, and benzyl butyl phthalate. Hair from a skin area (1.3 cm in diameter) on the back of male F344 rats was clipped, the 14(C)phthalate diester was applied in a dose of 157 mumol/kg, and the area of application was covered with a perforated cap. The rat was restrained and housed for 7 days in a metabolic cage that allowed separate collection of urine and feces. Urine and feces were collected every 24 hr, and the amount of (14)C excreted was taken as an index of the percutaneous absorption. At 24 hr, diethyl phthalate showed the greatest excretion (26%). As the length of the alkyl side chain increased, the amount of (14)C excreted in the first 24 hr decreased signficantly. The cumulative percentage dose excreted in 7 days was greatest for diethyl, dibutyl, and diisobutyl phthalate, about 50-60% of the applied (14)C; and intermediate (20-40%) for dimethyl, benzyl butyl, and dihexyl phthalate. Urine was the major route of excretion of all phthalate diesters except for diisodecyl phthalate. This compound was poorly absorbed and showed almost no urinary excretion. After 7 days, the percentage dose for each phthalate that remained in the body was minimal showed no specific tissue distribution. Most of the unexcreted dose remained in the area of application. These data show that the structure of the phthalate diester determines the degree of dermal absorption. Absorption maximized with diethyl phthalate and then decreased significantly as the alkyl side chain length increased.
PMID:2925020 Elsisi AE et al; Fundam Appl Toxicol 12 (1): 70-7 (1989)
DMP is readily absorbed from the skin, intestinal tract, the peritoneal cavity, and lung.
Bingham, E.; Cohrssen, B.; Powell, C.H.; Patty's Toxicology Volumes 1-9 5th ed. John Wiley & Sons. New York, N.Y. (2001)., p. V6 819
For more Absorption, Distribution and Excretion (Complete) data for DIMETHYL PHTHALATE (6 total), please visit the HSDB record page.
Intestinal extracts from man, ferrets and the baboon, as well as liver extracts from the latter 2 species, break down dimethyl phthalate to the monoester.
European Chemicals Bureau; IUCLID Dataset, Dimethyl phthalate (131-11-3) p.129 (2000 CD-ROM edition). Available from, as of April 25, 2008: https://esis.jrc.ec.europa.eu/
In vitro studies on metabolism of dimethylphthalate, dibutyl phathalate, di-n-octyl phthalate ... and diethylhexyl phthalate by rat liver and kidney liver homogenates have demonstrated that the lower the molecular weight of phthalate ester the faster the rate of metabolism. Rate of degradation of esters by rat kidney homogenates was relatively slow when compared with that by liver homogenates.
The Royal Society of Chemistry. Foreign Compound Metabolism in Mammals. Volume 6: A Review of the Literature Published during 1978 and 1979. London: The Royal Society of Chemistry, 1981., p. 340
Of a single dose of 120 mg dimethyl phthalate admin to rats by stomach tube, 44.6% was detected in the urine, consisting of 77.5% as the monomethyl ester with 14.4% as 0-phthalate acid and 8.1% as intact dimethyl phthalate.
European Chemicals Bureau; IUCLID Dataset, Dimethyl phthalate (131-11-3) p.129 (2000 CD-ROM edition). Available from, as of April 25, 2008: https://esis.jrc.ec.europa.eu/
... In a host-mediated mutagenesis assay, rats were injected ip with dimethyl phthalate (DMP) (2 g/kg body weight); urine was collected for 24 hr, extracted, and analyzed for ... phthalic acid-containing derivatives. The extracted urine ... contained an equivalent of 1.96 mg phthalate/mL urine. More than 97% of the phthalic acid-containing derivatives present in the extracted urine consisted of the nonmutagenic metabolite of DMP, monomethyl phthalate (MMP). In vitro experiments showed that rat liver homogenates hydrolyzed 93% of carbonyl-labeled (14)C-DMP (7.7 mM) to MMP in 2 hr and bound 0.07 nmol of ((14)C)phthalate/mg liver macromolecules. By contrast, rat epidermal homogenates metabolized only 5% and bound 38-fold higher levels of carbonyl-labeled (14)C-DMP (2.66 nmol/mg of macromolecules), with no detectable binding to nucleic acids. Compared to epidermis and plasma, liver had a fivefold higher rate of DMP monoesterase activity (1240 nmol/hr/mg protein), which, when inhibited by 67%, resulted in a 4.4-fold increase in phthalate-bound hepatic macromolecules (0.31 vs. 0.07 nmol of carbonyl-labeled (14)C-DMP/mg macromolecules). In addition to MMP, formaldehyde was produced during the metabolism of DMP by liver. When ethanol was used to inhibit the oxidation of DMP-derived methanol by hepatic homogenates, there resulted a 74% reduction in the accumulation of formaldehyde and similar reductions of 71 and 73% in the binding of methyl-labeled (14)C-DMP to nucleic acids and macromolecules. (Methyl-labeled, unlike carbonyl-labeled, (14)C-DMP yields a (14)C-labeled methanol when hydrolyzed.) These results indicate that the DMP diester ... binds to epidermal and hepatic macromolecules other than nucleic acids, and that although the rapid hepatic metabolism of DMP to its monoester (MMP) and methanol affords protection against higher levels of phthalate binding as well as against DMP-induced bacterial mutagenesis, it also oxidizes DMP-derived methanol to formaldehyde, a metabolite that binds macromolecules, including nucleic acids.
PMID:1709688 Kozumbo WJ, Rubin RJ; J Toxicol Environ Health 33 (1): 29-46 (1991)
For more Metabolism/Metabolites (Complete) data for DIMETHYL PHTHALATE (12 total), please visit the HSDB record page.


