



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0

USA (Orange Book)
0

Europe

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
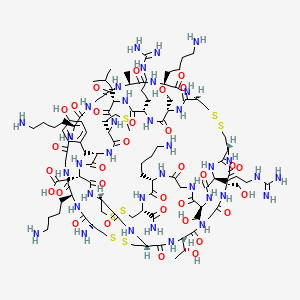

1. Cys-lys-gly-lys-gly-ala-lys-cys-ser-arg-leu-met-tyr-asp-cys-cys-thr-gly-ser-cys-arg-ser-gly-lys-cys-nh2
2. Leconotide
3. Omega-conopeptide Mviia
4. Omega-conotoxin M Viia
5. Omega-conotoxin Mviia
6. Omega-conotoxin Mviia, Conus Magus
7. Prialt
8. Snx 111
9. Snx-111
1. Prialt
2. Snx-111
3. 107452-89-1
4. Omega-conopeptide Mviia (conus)
5. Omega Conotoxin Mviia
6. 150770-63-1
7. Omega-conotoxin M Viia
8. H-cys-lys-gly-lys-gly-ala-lys-cys-ser-arg-leu-met-tyr-asp-cys-cys-thr-gly-ser-cys-arg-ser-gly-lys-cy
9. Conotoxin Mviia
10. Omega-conotoxin Mviia, Conus Magus
11. Drg-0250
12. Ziconotide [usan:inn]
13. Unii-7i64c51o16
14. Hsdb 7609
15. O-conotoxin Mviia
16. Mfcd00145036
17. Ziconotide Polyacetate
18. Ctx Mviia
19. Conotoxin Mviia_smartox
20. .omega.-conotoxin M Viia
21. Omega-conotoxin M Viia (reduced), Cyclic (1-16),(8-20),(15-25)-tris(disulfide)
22. Gtpl2536
23. Chembl4594214
24. Dtxsid60883174
25. Chebi:135912
26. 08con001
27. Hy-p0062
28. Akos015994641
29. 7i64c51o16
30. Hs-2032
31. Omega-conotoxin Mviia, >=95% (hplc)
32. Cs-0015087
33. Y-100004
34. L-cysteinyl-l-lysylglycyl-l-lysylglycyl-l-alanyl-l-lysyl-l-cysteinyl-l-seryl-l-arginyl-l-leucyl-l-methionyl-l-tyrosyl-l-alpha-aspartyl-l-cysteinyl-l-cysteinyl-l-threonylglycyl-l-seryl-l-cysteinyl-l-arginyl-l-serylglycyl-l-lysyl-l-cysteinamide Cyclic (1-16),(8-20),(15-25)-tris(disulfide)
| Molecular Weight | 2639.2 g/mol |
|---|---|
| Molecular Formula | C102H172N36O32S7 |
| XLogP3 | -14 |
| Hydrogen Bond Donor Count | 42 |
| Hydrogen Bond Acceptor Count | 46 |
| Rotatable Bond Count | 40 |
| Exact Mass | 2638.1016905 g/mol |
| Monoisotopic Mass | 2637.0983357 g/mol |
| Topological Polar Surface Area | 1310 Ų |
| Heavy Atom Count | 177 |
| Formal Charge | 0 |
| Complexity | 5480 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 22 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Ziconotide is used intrathecally for the management of severe chronic pain in patients who are intolerant of or do not obtain adequate pain relief from other therapies (e.g., systemic analgesics, adjunctive therapies, intrathecal morphine therapy) when intrathecal therapy is warranted.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 2241
/BOXED WARNING/ WARNING: NEUROPSYCHIATRIC ADVERSE REACTIONS. Prialt is contraindicated in patients with a preexisting history of psychosis. Severe psychiatric symptoms and neurological impairment may occur during treatment with Prialt. Monitor all patients frequently for evidence of cognitive impairment, hallucinations, or changes in mood or consciousness. Discontinue Prialt therapy in the event of serious neurological or psychiatric signs or symptoms.
US Natl Inst Health (NIH); DailyMed. Current Medication Information. Prialt (Ziconotide) Injection, Solution. (Updated February 2013). Available from, as of April 24, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5449ca98-efb8-4c3b-8756-747b2349a472
Meningitis has occurred in patients receiving ziconotide, principally in individuals receiving therapy via an external microinfusion device and catheter. Meningitis may occur secondary to inadvertent contamination of the microinfusion device or as a result of CSF seeding caused by hematogenous or direct spread from an infected pump pocket or catheter tract. Patients should be monitored for signs and symptoms of meningitis (e.g., fever, headache, stiff neck, altered mental status, nausea or vomiting, seizures). Preparation of ziconotide solution and filling of the drug reservoir should be performed under aseptic conditions by trained and qualified personnel. If meningitis is suspected (especially in immunocompromised patients) or is confirmed, appropriate measures (CSF culture, anti-infective therapy, removal of the microinfusion device and catheter) should be initiated.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 2241
Use of Prialt has been associated with CNS-related adverse events, including psychiatric symptoms, cognitive impairment, and decreased alertness/unresponsiveness. For the 1254 patients treated /in clinical trials/, the following cognitive adverse event rates were reported: confusion (33%), memory impairment (22%), speech disorder (14%), aphasia (12%), thinking abnormal (8%), and amnesia (1%). Cognitive impairment may appear gradually after several weeks of treatment. The PRIALT dose should be reduced or discontinued if signs or symptoms of cognitive impairment develop, but other contributing causes should also be considered. The various cognitive effects of Prialt are generally reversible within 2 weeks after drug discontinuation. The medians for time to reversal of the individual cognitive effects ranged from 3 to 15 days. The elderly (> or = 65 years of age) are at higher risk for confusion.
US Natl Inst Health (NIH); DailyMed. Current Medication Information. Prialt (Ziconotide) Injection, Solution. Feb, 2008. Available from, as of May 27, 2008: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=6893
Cognitive impairment (e.g., confusion, memory impairment, speech disorder, aphasia, abnormal thinking, amnesia) has been reported in patients receiving ziconotide. Cognitive impairment may appear gradually over several weeks and generally is reversible following discontinuance of the drug. If cognitive impairment develops, the dose of ziconotide should be reduced or the drug discontinued; other causes that could contribute to cognitive impairment should be considered.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 2241
For more Drug Warnings (Complete) data for ZICONOTIDE (17 total), please visit the HSDB record page.
Ziconotide is indicated for the management of severe chronic pain in patients refractory to other treatments, and for whom intrathecal therapy is warranted.
Ziconotide is indicated for the treatment of severe, chronic pain in patients who require intrathecal (IT) analgesia.
Ziconotide inhibits N-type calcium channels involved in nociceptive signalling, primarily in the dorsal horn of the spinal cord. Although binding is reversible, careful dosing is required to ensure therapeutic effects while minimizing adverse effects, and ziconotide has been described as possessing a narrow therapeutic window. Patients taking ziconontide may experience cognitive and neuropsychiatric symptoms, reduced levels of consciousness, and elevated serum creatine kinase levels. In addition, ziconotide may increase the risk of infection, including serious cases of meningitis. Patients who withdraw from opiates for ziconotide initiation are advised to taper off the dose.
Analgesics, Non-Narcotic
A subclass of analgesic agents that typically do not bind to OPIOID RECEPTORS and are not addictive. Many non-narcotic analgesics are offered as NONPRESCRIPTION DRUGS. (See all compounds classified as Analgesics, Non-Narcotic.)
Calcium Channel Blockers
A class of drugs that act by selective inhibition of calcium influx through cellular membranes. (See all compounds classified as Calcium Channel Blockers.)
Neuroprotective Agents
Drugs intended to prevent damage to the brain or spinal cord from ischemia, stroke, convulsions, or trauma. Some must be administered before the event, but others may be effective for some time after. They act by a variety of mechanisms, but often directly or indirectly minimize the damage produced by endogenous excitatory amino acids. (See all compounds classified as Neuroprotective Agents.)
N02BG08
N - Nervous system
N02 - Analgesics
N02B - Other analgesics and antipyretics
N02BG - Other analgesics and antipyretics
N02BG08 - Ziconotide
Absorption
Ziconotide administered intrathecally over one hour in doses between 1 and 10 mcg produced calculated AUC values between 83.6-608 ng\*h/mL and Cmax between 16.4-132 ng/mL; these values are approximately dose-proportional. Given the intrathecal administration and low membrane permeability due to its size, ziconotide is expected to remain primarily in the CSF; plasma levels, where detected, remain constant up to nine months following administration.
Route of Elimination
A small fraction of intravenous ziconotide (< 1%) is recovered in urine.
Volume of Distribution
In patients administered 1-10 mcg intrathecal ziconotide over one hour, the apparent volume of distribution was calculated as 155 263 mL; this value is roughly equivalent to the expected CSF volume. Although intravenous administration is not indicated, intravenous administration of between 0.3-10 mcg/kg/day ziconotide resulted in an apparent volume of distribution of 30,460 6366 mL.
Clearance
Ziconotide CSF clearance is 0.38 0.56 mL/min while plasma clearance is 270 44 mL/min.
Ziconotide reached a maximal brain concentration of between 0.003 and 0.006% of the injected material per gram of tissue at 3-20 min after i.v. injection, and this decayed to below 0.001%/g after 2 hr. ... The peptide was perfused through in vivo dialysis probes implanted into the hippocampus. Image analysis and serial sectioning showed that diffusion of Ziconotide in the extracellular fluid around the dialysis probe was minimal, with the peptide located within 1 mm of the probe after 2 hr. ... Passage from blood to brain was also verified by in situ perfusion through the carotid artery. A statistically greater amount of radioactivity was found to cross the BBB after perfusion of radioiodinated Ziconotide compared to (14)C inulin.
PMID:10822104 Newcomb R et al; Peptides 21 (4): 491-501(2000)
The pharmacokinetics and pharmacodynamics of ziconotide were assessed over a 48-hour period following intrathecal (i.t.) administration (1, 5, 7.5, or 10 ug) to 22 patients with chronic, nonmalignant pain. Plasma and cerebrospinal fluid samples were obtained over a 24-hour period. Analgesic efficacy was monitored using Visual Analog Scale of Pain Intensity (VASPI) and Category Pain Relief Scores (CPRS) measurements. Pharmacokinetic (PK) parameters were calculated by noncompartmental methods. Plasma ziconotide data were insufficient for pharmacokinetic calculations. In cerebrospinal fluid, the median half-life of ziconotide was 4.5 hours. The median cerebrospinal fluid clearance and volume of distribution were 0.26 mL/min and 99 mL, respectively. Cerebrospinal fluid pharmacokinetics of ziconotide were linear, based on cumulative exposure and peak cerebrospinal fluid concentrations. A dose-related analgesia was observed. ...
PMID:12817525 Wermeling D et al; J Clin Pharmacol 43 (6): 624-36 (2003)
Intrathecal administration of ziconotide results in little systemic exposure. Following passage from the CSF into the systemic circulation, ziconotide is expected to be degraded to peptide fragments and their constituent amino acids by endopeptidases and exopeptidases present in most organs.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 2242
Ziconotide is about 50% bound to human plasma proteins. The mean cerebrospinal fluid (CSF) volume of distribution (Vd) of ziconotide following intrathecal administration approximates the estimated total CSF volume (140 mL).
US Natl Inst Health (NIH); DailyMed. Current Medication Information. Prialt (Ziconotide) Injection, Solution. Feb, 2008. Available from, as of May 27, 2008: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=6893
For more Absorption, Distribution and Excretion (Complete) data for ZICONOTIDE (6 total), please visit the HSDB record page.
Ziconotide is expected to be processed by various peptidases upon entering systemic circulation; no detailed information on ziconotide metabolism has been reported.
Ziconotide is rapidly distributed and/or metabolized in spinal cerebrospinal fluid (CSF) after intrathecal administration, followed by relatively rapid mass transport of the product from the CSF into the plasma. The relative contributions of mass transport, within and outside the spinal cord, and metabolism within it, are unclear. There is certainly evidence for rapid transport into the blood and metabolism within the spinal cord is likely to have a significant role. Following entry into the blood, the compound is quickly metabolized by normal proteolytic mechanisms, eventually to its constituent amino acids; it can be assumed that these will be further metabolized or incorporated into proteins by normal processes.
European Medicines Agency (EMEA); European Public Assessment Report (EPARs) for Authorized Medicinal Products for Human Use; Scientific Discussion; p.6 (February 28, 2008). Available from, as of May 28, 2008: https://www.emea.europa.eu/humandocs/PDFs/EPAR/Prialt/14122704en6.pdf
Ziconotide is cleaved by endopeptidases and exopeptidases at multiple sites on the peptide. Following passage from the cerebrospinal fluid (CSF) into the systemic circulation during continuous IT administration, ziconotide is expected to be susceptible to proteolytic cleavage by various ubiquitous peptidases/proteases present in most organs (e.g., kidney, liver, lung, muscle, etc.), and thus readily degraded to peptide fragments and their individual constituent free amino acids. Human and animal CSF and blood exhibit minimal hydrolytic activity toward ziconotide in vitro. The biological activity of the various expected proteolytic degradation products of ziconotide has not been assessed.
US Natl Inst Health (NIH); DailyMed. Current Medication Information. Prialt (Ziconotide) Injection, Solution. Feb, 2008. Available from, as of May 27, 2008: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=6893
In patients administered 1-10 mcg intrathecal ziconotide over one hour, the elimination half-life was calculated as 4.6 0.9 hr. Although intravenous administration is not indicated, intravenous administration of between 0.3-10 mcg/kg/day ziconotide resulted in an elimination half-life of 1.3 0.3 hr.
The terminal half-life of ziconotide in cerebrospinal fluid after an intrathecal administration was around 4.6 hours (range 2.9-6.5 hours).
US Natl Inst Health (NIH); DailyMed. Current Medication Information. Prialt (Ziconotide) Injection, Solution. Feb, 2008. Available from, as of May 27, 2008: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=6893
Nociceptive pain signalling is a complex processing pathway involving peripheral nociceptors, primary afferent nerve fibres, and downstream CNS neurons located in the spinal cord. Voltage-gated calcium channels (VGCCs) are important regulatory components of neural signalling and include the N-type (Cav2.2) heteromultimeric high-voltage type calcium channels. Chronic pain conditions, including inflammatory and neuropathic pain, often involve the aberrant upregulation of VGCC activity through various cellular mechanisms, which can lead to allodynia and hyperalgesia. Specifically, N-type channel activation in lightly myelinated A- and C-fibres is known to mediate the release of neurotransmitters substance P (SP), calcitonin gene-related peptide (CGRP), and glutamate, which influence downstream neural activation and pain perception. In addition, SP and CGRP induce inflammation, potentially exacerbating pre-existing inflammatory chronic pain. Ziconotide belongs to the -conotoxin class of neurotoxic peptides derived from the cone snail _Conus magus_ which are capable of inhibiting N-type VGCCs. Although the exact mechanism is yet to be elucidated, it is thought that -conotoxins function through direct occlusion of the ion pore to prevent calcium translocation across the membrane. Additional studies involving expression of chimeric subunits and molecular modelling suggest that insertion of the ziconotide Met12 residue into a hydrophobic pocket formed by Ile300, Phe302, and Leu305 of Cav2.2 increases binding and may be associated with toxic adverse effects.
/Ziconotide/ is a N-type calcium channel blocker (NCCB). Voltage-sensitive calcium channel (VSCC) conduction plays a major role in the transmission of pain. The N-type VSCC's are found in high concentrations in the dorsal root ganglion cells responsible for the spinal processing of pain. Ziconotide selectively and reversibly binds to and blocks these channels without interacting with other ion channels or cholinergic, monoaminergic or mu and delta-opioid receptors. Ziconotide thus inhibits the spinal signalling of pain.
European Medicines Agency (EMEA); European Public Assessment Report (EPARs) for Authorized Medicinal Products for Human Use; Scientific Discussion; p.1 (February 28, 2008). Available from, as of May 28, 2008: https://www.emea.europa.eu/humandocs/PDFs/EPAR/Prialt/14122704en6.pdf


