



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0

USA (Orange Book)
0

Europe

Canada
0

Australia
0

South Africa
Uploaded Dossiers
U.S. Medicaid
0
Annual Reports
0
0
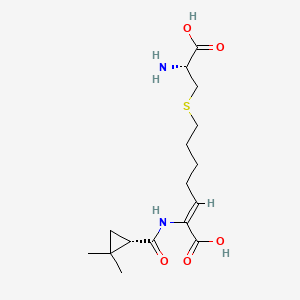

1. Cilastatin Monosodium Salt
2. Cilastatin Sodium
3. Mk 0791
4. Mk 791
5. Mk-791
6. Mk0791
7. Mk791
8. Monosodium Salt, Cilastatin
9. Salt, Cilastatin Monosodium
10. Sodium, Cilastatin
1. 82009-34-5
2. Cilastatina
3. Cilastatine
4. Cilastatinum
5. Mk-791
6. Cilastatin (inn)
7. Mk0791
8. Chebi:3697
9. Cilastatin Acid
10. 141a6amn38
11. Mk 0791
12. (2z)-7-{[(2r)-2-amino-2-carboxyethyl]sulfanyl}-2-{[(1s)-2,2-dimethylcyclopropyl]formamido}hept-2-enoic Acid
13. Cilastatine [french]
14. Cilastatinum [latin]
15. Cilastatin [inn]
16. Cilastatina [spanish]
17. Cilastatin [inn:ban]
18. (z)-(s)-6-carboxy-6-[(s)-2,2-dimethylcyclopropanecarboxamido]hex-5-enyl-l-cysteine
19. (z)-7-(((r)-2-amino-2-carboxyethyl)thio)-2-((s)-2,2-dimethylcyclopropane-1-carboxamido)hept-2-enoic Acid
20. Cilastin
21. (l)-7-(2-amino-2-carboxy-ethylsulfanyl)-2-[(2,2-dimethyl-cyclopropanecarbonyl)-amino]-hept-2-enoic Acid
22. (z)-7-((r)-2-amino-2-carboxy-ethylsulfanyl)-2-[((s)-2,2-dimethyl-cyclopropanecarbonyl)-amino]-hept-2-enoic Acid
23. Ncgc00181346-01
24. Einecs 279-875-8
25. Unii-141a6amn38
26. Mk791
27. (z)-7-[(2s)-2-amino-3-hydroxy-3-oxopropyl]sulfanyl-2-[[(1s)-2,2-dimethylcyclopropanecarbonyl]amino]hept-2-enoic Acid
28. Cilastatin [mi]
29. 81129-83-1
30. Cilastatin [vandf]
31. Chembl766
32. Cilastatin [who-dd]
33. Schembl37051
34. 2-heptenoic Acid, 7-((2-amino-2-carboxyethyl)thio)-2-(((2,2-dimethylcyclopropyl)carbonyl)amino)-, (r-(r*,s*-(z)))-
35. Bidd:gt0782
36. Gtpl5166
37. Dtxsid8048238
38. Ex-a4954
39. Hy-a0166
40. Zinc4095696
41. Ac-268
42. Bdbm50367502
43. Mfcd00867379
44. Akos015962144
45. Cs-8177
46. Db01597
47. (2z)-7-{[(2r)-2-amino-2-carboxyethyl]sulfanyl}-2-({[(1s)-2,2-dimethylcyclopropyl]carbonyl}amino)hept-2-enoic Acid
48. Ds-11972
49. A16977
50. C01675
51. D07698
52. A840230
53. Q418201
54. Sr-01000781260
55. Sr-01000781260-3
56. (z)-7-[(2r)-2-azanyl-3-oxidanyl-3-oxidanylidene-propyl]sulfanyl-2-[[(1s)-2,2-dimethylcyclopropyl]carbonylamino]hept-2-enoic Acid
57. (z)-7-[[(2r)-2-amino-2-carboxyethyl]thio]-2-[[[(1s)-2,2-dimethylcyclopropyl]-oxomethyl]amino]-2-heptenoic Acid
58. 2-heptenoic Acid, 7-[[(2r)-2-amino-2-carboxyethyl]thio]-2-[[[(1s)-2,2-dimethylcyclopropyl]carbonyl]amino]-, (2z)-
| Molecular Weight | 358.5 g/mol |
|---|---|
| Molecular Formula | C16H26N2O5S |
| XLogP3 | -1 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 11 |
| Exact Mass | 358.15624311 g/mol |
| Monoisotopic Mass | 358.15624311 g/mol |
| Topological Polar Surface Area | 155 Ų |
| Heavy Atom Count | 24 |
| Formal Charge | 0 |
| Complexity | 519 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Cilastatin is indicated, in combination with [imipenem] with or without [relebactam], for the treatment of bacterial infections including respiratory, skin, bone, gynecologic, urinary tract, and intra-abdominal as well as septicemia and endocarditis.
FDA Label
Cilastatin is a chemical compound which inhibits the human enzyme dehydropeptidase. Renal Dehydropeptidase degrades the antibiotic [imipenem]. Cilastatin is therefore combined intravenously with imipenem in order to protect it from dehydropeptidase and prolong its antibacterial effect. However, cilastatin in and of itself does not have any antibacterial activity. The increased renal excretion of unchanged imipenem appears to prevent proximal tubular necrosis associated with high doses of imipenem.
Protease Inhibitors
Compounds which inhibit or antagonize biosynthesis or actions of proteases (ENDOPEPTIDASES). (See all compounds classified as Protease Inhibitors.)
Route of Elimination
Cilastatin is reported by official FDA labeling to be 70% excreted in the urine, however published literature has reported values as high as 98%.
Volume of Distribution
Cilastatin has a volume of distribution of 14.6-20.1L.
Clearance
Cilastatin has a total clearance of 0.2 L/h/kg and a renal clearance of 0.10-0.16 L/h/kg.
The half-life of cilastatin is approximately 1h.
Cilastatin is a renal dehydropeptidase-I inhibitor. Since the antibiotic, imipenem, is hydrolyzed by dehydropeptidase-I, which resides in the brush border of the renal tubule, cilastatin is administered with imipenem to block the metabolism of imipenem.


