
Synopsis
Synopsis
0
VMF
0
FDA Orange Book
0
Australia
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
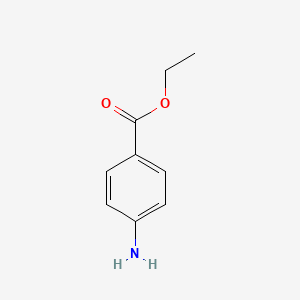

1. 4-aminobenzoic Acid Ethyl Ester
2. Acetate, Benzocaine
3. Americaine
4. Anaesthesin
5. Anesthesin
6. Bensokain
7. Benzocaine Acetate
8. Benzocaine Formate
9. Benzocaine Hydrobromide
10. Benzocaine Hydrochloride
11. Benzocaine Methanesulfonate
12. Ethoform
13. Ethyl Aminobenzoate
14. Formate, Benzocaine
15. Hydrobromide, Benzocaine
16. Hydrochloride, Benzocaine
17. Methanesulfonate, Benzocaine
1. Ethyl 4-aminobenzoate
2. 94-09-7
3. Ethyl Aminobenzoate
4. Ethyl P-aminobenzoate
5. Americaine
6. Anaesthesin
7. Parathesin
8. Norcaine
9. Parathesine
10. Anesthesin
11. 4-aminobenzoic Acid Ethyl Ester
12. Ethoform
13. Orthesin
14. Amben Ethyl Ester
15. P-carbethoxyaniline
16. Anaesthin
17. Anestezin
18. Anesthesine
19. Anesthone
20. Dermoplast
21. Hurricaine
22. Identhesin
23. Keloform
24. Norcain
25. Topcaine
26. Baby Anbesol
27. Anaesthan-syngala
28. Ora-jel
29. Benzocainum
30. Solu H
31. P-ethoxycarboxylic Aniline
32. 4-carbethoxyaniline
33. Benzoic Acid, 4-amino-, Ethyl Ester
34. P-(ethoxycarbonyl)aniline
35. Ethyl P-aminophenylcarboxylate
36. Dextran Sulfate Sodium
37. 9011-18-1
38. Benzocaina
39. Ethyl Paba
40. 4-(ethoxycarbonyl)aniline
41. P-aminobenzoic Acid Ethyl Ester
42. Aethoform
43. Benzoic Acid, P-amino-, Ethyl Ester
44. Ethyl4-aminobenzoate
45. 4-aminobenzoic Acid, Ethyl Ester
46. 4-amino-benzoic Acid Ethyl Ester
47. Ethylester Kyseliny P-aminobenzoove
48. Ethyl-p-aminobenzoate
49. Flavamed
50. Subcutin
51. P-aminobenzoic Acid, Ethyl Ester
52. Chebi:116735
53. Nsc-4688
54. Mfcd00007892
55. Nsc-41531
56. U3rsy48jw5
57. Ar01
58. 4-aminobenzoic Acid-ethyl Ester
59. Anaesthesinum
60. Ethoforme
61. Norcainum
62. Ar-01
63. Ethylis Aminobenzoas
64. Cas-94-09-7
65. Ncgc00016352-01
66. Anestezin [russian]
67. Chloraseptic
68. Dextran Sulfate Sodium Salt
69. Otocain
70. Outgro
71. Dsstox_cid_1804
72. Wln: Zr Dvo2
73. Dsstox_rid_76338
74. Aethylium Paraminobenzoicum
75. Benzocainum [inn-latin]
76. Dsstox_gsid_21804
77. Benzocaina [inn-spanish]
78. Ethyl Aminobenzoate (van)
79. Caswell No. 430a
80. Finafta
81. Parathesin (tn)
82. Smr000059025
83. Ethylaminobenzoate-4
84. H-4-abz-oet
85. Hsdb 7225
86. Ethylester Kyseliny P-aminobenzoove [czech]
87. Benzocaine (usp/inn)
88. Sr-05000001573
89. Einecs 202-303-5
90. Nsc 41531
91. P-aminobenzoic Ethyl Ester
92. Unii-u3rsy48jw5
93. Epa Pesticide Chemical Code 097001
94. Brn 0638434
95. Benzoak
96. Benzocaine [usp:inn:ban]
97. Vagisil
98. Ai3-02081
99. Benzocaine Usp
100. Diet Ayds
101. Orabase-b
102. Slim Mint Gum
103. Prestwick_991
104. 4-carboethoxyaniline
105. Ethyl 4aminobenzoate
106. Ethyl 4-aminobenzate
107. Ethyl 4-aminobezoate
108. Outgro (salt/mix)
109. Anbesol (salt/mix)
110. Vagisil (salt/mix)
111. Ethyl-4-aminobenzoate
112. P-ethoxycarbonylaniline
113. 4-ethoxycarbonylaniline
114. Ethyl P-amino-benzoate
115. Ethyl-p-amino-benzoate
116. Ethyl Aminobenzoic Acid
117. Cough-x (salt/mix)
118. Spectrum_000074
119. Ethyl 4-amino-benzoate
120. Benzocaine [mi]
121. Ethyl 4-(amino)benzoate
122. Benzocaine [inn]
123. Ethyl P-aminobenzoic Acid
124. Prestwick0_000712
125. Prestwick1_000712
126. Prestwick2_000712
127. Prestwick3_000712
128. Spectrum2_000117
129. Spectrum3_000314
130. Spectrum4_000249
131. Spectrum5_000860
132. Benzocaine [hsdb]
133. Ethyl 4-aminobenzoic Acid
134. Benzocaine [vandf]
135. Epitope Id:114084
136. Ethyl Paba [inci]
137. Benzocaine [mart.]
138. Benzocaine [usp-rs]
139. Benzocaine [who-dd]
140. Benzocaine [who-ip]
141. Sampl3, G4
142. Ethyl Aminobenzoate (jp17)
143. Oprea1_750694
144. Oprea1_827402
145. Schembl25100
146. Bspbio_000923
147. Bspbio_001908
148. Kbiogr_000658
149. Kbioss_000474
150. 4-(ethoxycarbonyl)phenylamine
151. 4-14-00-01129 (beilstein Handbook Reference)
152. Ae-562/40377256
153. Mls001331704
154. Mls002153970
155. Divk1c_000932
156. Ethyl 4-aminobenzoate, 98%
157. Spectrum1500139
158. (p-(ethoxycarbonyl)phenylamine
159. Ethyl P-aminobenzenecarboxylate
160. Spbio_000134
161. Spbio_002844
162. Bpbio1_001017
163. Chembl278172
164. Dtxsid8021804
165. Dextran Sulfate Sodium (dst-h)
166. Ethyl Para Amino Benzoate
167. Hms502o14
168. Kbio1_000932
169. Kbio2_000474
170. Kbio2_003042
171. Kbio2_005610
172. Kbio3_001408
173. P-amino Benzoic Acid Ethyl Ester
174. 4-amino Benzoic Acid Ethyl Ester
175. Benzocaine [ep Monograph]
176. Ethyl Aminobenzoate [jan]
177. Nsc4688
178. Dextran Sulfate Sodium (kmds-h)
179. Ninds_000932
180. Bdbm197282
181. Benzocaine [usp Monograph]
182. Dextran Sulfate Sodium (ds-m-1)
183. Hms1570o05
184. Hms1920g09
185. Hms2091m11
186. Hms2097o05
187. Hms2233h21
188. Hms3371d08
189. Hms3652h13
190. Hms3714o05
191. Hms3885b11
192. Pharmakon1600-01500139
193. Benzocainum [who-ip Latin]
194. Component Of Tympagesic (salt/mix)
195. Cs-b0934
196. Hy-y0258
197. Nsc41531
198. Str01509
199. Tox21_110391
200. Tox21_301149
201. Ccg-38918
202. Nsc755909
203. S4210
204. Stk043620
205. Zinc12358719
206. Akos000119763
207. Benzocaine 1.0 Mg/ml In Acetonitrile
208. Benzocaine 100 Microg/ml In Methanol
209. Tox21_110391_1
210. Ac-8127
211. Db01086
212. Dextran, Hydrogen Sulfate, Sodium Salt
213. Nsc-755909
214. Idi1_000932
215. Benzocaine, Purum, >=99.0% (hplc)
216. Benzocaine, Tested According To Ph.eur.
217. Ncgc00016352-02
218. Ncgc00016352-03
219. Ncgc00016352-04
220. Ncgc00016352-07
221. Ncgc00094598-01
222. Ncgc00094598-02
223. Ncgc00255047-01
224. Ethyl 4-aminobenzoate, 98.0-101.0%
225. Sbi-0051293.p003
226. Component Of Solarcaine Aerosol (salt/mix)
227. A0271
228. Ab00051923
229. Bb 0258778
230. Ft-0624536
231. Ft-0625762
232. Ft-0662548
233. Sw197074-3
234. A14560
235. C07527
236. D00552
237. Ab00051923_09
238. Ab00051923_10
239. Component Of Anbesol Maximum Strength (salt/mix)
240. A860898
241. A930377
242. Aminobenzoic Acid Impurity B [ep Impurity]
243. Q422745
244. Ethyl 4-aminobenzoate, Saj First Grade, >=99.0%
245. Q-200688
246. Sr-05000001573-1
247. Sr-05000001573-3
248. Brd-k75466013-001-05-2
249. Brd-k75466013-001-08-6
250. Ethyl 4-aminobenzoate, Vetec(tm) Reagent Grade, 98%
251. F2190-0448
252. 4-aminobenzoic Acid-ethyl Ester 1000 Microg/ml In Methanol
253. Benzocaine, European Pharmacopoeia (ep) Reference Standard
254. Benzocaine, United States Pharmacopeia (usp) Reference Standard
255. Benzocaine, Pharmaceutical Secondary Standard; Certified Reference Material
| Molecular Weight | 165.19 g/mol |
|---|---|
| Molecular Formula | C9H11NO2 |
| XLogP3 | 1.9 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 3 |
| Exact Mass | 165.078978594 g/mol |
| Monoisotopic Mass | 165.078978594 g/mol |
| Topological Polar Surface Area | 52.3 Ų |
| Heavy Atom Count | 12 |
| Formal Charge | 0 |
| Complexity | 151 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Indicated for the relief of: canker sores, cold sores, or fever blisters: benzocaine (gel and topical solution); gingival or oral mucosal pain (i.e., pain caused by mouth or gum irritation, inflammation, lesions, or minor dental procedures): benzocaine (gel, dental paste, lozenges, and topical solution); dental prosthetic pain (i.e., pain or irritation caused by dentures or other dental or orthodontic appliances): benzocaine (dental paste, gel ointment, and topical solution); teething pain: benzocaine (7.5% and 10% gel); and toothache: benzocaine (10% and 20% gel and topical solution).
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 153
Indicated to suppress the gag reflex and/or other laryngeal and esophageal reflexes to facilitate dental examination or procedures (including oral surgery), endoscopy, or intubation: benzocaine (gel, topical aerosol, and topical solution). /Included in US product labeling/
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 153
Indicated to provide topical anesthesia of accessible mucous membranes prior to examination, endoscopy or instrumentation, or other procedures involving the: esophagus: benzocaine (gel and topical solution)); larynx: benzocaine (gel and topical solution); mouth, In dental procedures and oral surgery: benzocaine (gel, topical aerosol, and topical solution); nasal cavity: benzocaine (gel); pharynx or throat: benzocaine (gel, topical aerosol, and topical solution); rectum: benzocaine (gel); respiratory tract or trachea: benzocaine (gel, topical aerosol, and topical solution); urinary tract: benzocaine (gel); and vagina: benzocaine (gel).
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 153
Anesthetic (local).
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th Edition, Whitehouse Station, NJ: Merck and Co., Inc., 2001., p. 186
(VET): Local (usually surface) anesthetic.
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th Edition, Whitehouse Station, NJ: Merck and Co., Inc., 2001., p. 186
Infants and the elderly were more likely to develop toxic methemoglobinemia after benzocaine exposure. Other risk factors included genetic reductase deficiencies, exposure to high doses of anesthetic, and presence of denuded skin and mucous membranes. Because of the potential for severe complications, methemoglobinemia should be corrected promptly in compromised patients and those with toxic benzocaine concentrations. The possibility of masking symptoms during general anesthesia carries special risk of use of this agent in the preanesthesia setting.
PMID:8069004 Rodriguez LF et al; Ann Pharmacother 28 (5): 643-9 (1994)
Use of otic anesthetics may mask symptoms of a fulminating middle ear infection (acute otitis media). Otic solutions containing benzocaine should not be used in the presence of a perforated tympanic membrane.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2004. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2004 (Plus Supplements)., p. 2695
When applied topically as recommended, benzocaine is relatively nontoxic, however, sensitization may occur.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2004. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2004 (Plus Supplements)., p. 3411
When used as a male genital desensitizer, benzocaine generally does not adversely affect orgasm in female sexual partners and does not appear to anesthetize the clitoris or vagina.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2004. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2004 (Plus Supplements)., p. 3411
For more Drug Warnings (Complete) data for BENZOCAINE (9 total), please visit the HSDB record page.
Benzocaine is indicated for local anesthesia in dentistry, minor trauma, and as preparation for infiltrative anesthesia. Benzocaine products are indicated for topical anesthesia in a wide variety of conditions including skin irritation, oral pain, and hemorrhoids.
Treatment of oropharyngeal pain
Benzocaine is indicated for use as a topical anesthetic. It has a duration of action of approximately 10 minutes and a wide therapeutic window. Patients should be counselled regarding the risks of methemoglobinemia.
Anesthetics, Local
Drugs that block nerve conduction when applied locally to nerve tissue in appropriate concentrations. They act on any part of the nervous system and on every type of nerve fiber. In contact with a nerve trunk, these anesthetics can cause both sensory and motor paralysis in the innervated area. Their action is completely reversible. (From Gilman AG, et. al., Goodman and Gilman's The Pharmacological Basis of Therapeutics, 8th ed) Nearly all local anesthetics act by reducing the tendency of voltage-dependent sodium channels to activate. (See all compounds classified as Anesthetics, Local.)
C05AD03
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
C - Cardiovascular system
C05 - Vasoprotectives
C05A - Agents for treatment of hemorrhoids and anal fissures for topical use
C05AD - Local anesthetics
C05AD03 - Benzocaine
D - Dermatologicals
D04 - Antipruritics, incl. antihistamines, anesthetics, etc.
D04A - Antipruritics, incl. antihistamines, anesthetics, etc.
D04AB - Anesthetics for topical use
D04AB04 - Benzocaine
N - Nervous system
N01 - Anesthetics
N01B - Anesthetics, local
N01BA - Esters of aminobenzoic acid
N01BA05 - Benzocaine
R - Respiratory system
R02 - Throat preparations
R02A - Throat preparations
R02AD - Anesthetics, local
R02AD01 - Benzocaine
Branchial and urinary elimination of benzocaine residues was evaluated in adult rainbow trout, Oncorhynchus mykiss, given a single dorsal aortic dose of 14(C)-benzocaine hydrochloride. Branchial elimination of benzocaine residues was rapid and accounted for 59.2% of the dose during the first 3 h after dosing. Renal elimination of radioactivity was considerably slower; the kidney excreted 2.7% dose within 3 h and 9.0% within 24 hr. Gallbladder bile contained 2.0% dose 24 hr after injection. Of the radioactivity in radiochromatograms from water taken 3 min after injection, 87.3% was benzocaine and 12.7% was N-acetylated benzocaine. After 60 min, 32.7% was benzocaine and 67.3% was N-acetylated benzocaine. Of the radioactivity in radiochromatograms from urine taken 1 hr after dosing, 7.6% was para-aminobenzoic acid, 59.7% was N-acetylated para-aminobenzoic acid, 19.5% was benzocaine, and 8.0% was N-acetylated benzocaine. The proportion of the radioactivity in urine changed with time so that by 20 hr, 1.0% was para-aminobenzoic acid and 96.6% was N-acetylated para-aminobenzoic acid. Benzocaine and a more hydrophobic metabolite, N-acetylated benzocaine, were eliminated primarily through the gills; renal and biliary pathways were less significant elimination routes for benzocaine residues.
PMID:1897251 Meinertz JR et al; Xenobiotica 21 (4): 525-33 (1991)
Benzocaine undergoes ester hydrolysis to form 4-aminobenzoic acid, acetylation to form acetylbenzocaine, or N-hydroxylation to form benzocaine hydroxide. 4-aminobenzoic acid can be acetylated or acetylbenzocaine can undergo ester hydrolysis to form 4-acetaminobenzoic acid.
The effect of dose and enzymatic inhibition on the percutaneous absorption and metabolism of benzocaine was studied in vitro in the hairless guinea pig. At the dose level of 2 ug/sq cm, benzocaine was rapidly absorbed and extensively metabolized (80%) by acetyltransferase. As the applied dose of benzocaine was increased to 40 and 200 ug/sq cm, N-acetylation of benzocaine decreased to 44 and 34%, respectively, suggesting saturation of the acetyltransferase system. Total 14(C) absorption after benzocaine application was not significantly different between control and enzyme-inhibited skin and therefore does not appear to be affected by the extent of benzocaine metabolism during percutaneous penetration. Skin provides a significant first-pass metabolic effect for therapeutic doses of percutaneously absorbed benzocaine, and the primary metabolite formed, acetylbenzocaine, is biologically active.
PMID:8737920 Kraeling ME et al; Skin Pharmacol 9 (3): 221-30 (1996)
Benzocaine diffuses into nerve cells where it binds to sodium channels, preventing the channels from opening, and blocking the influx of sodium ions. Nerve cells unable to allow sodium into cells cannot depolarize and conduct nerve impulses.
Benzocaine reversibly stabilizes the neuronal membrane with decreases its permeability to sodium ions. Depolarization of the neuronal membrane is inhibited thereby blocking the initiation and conduction of nerve impulses.
Physicians Desk Reference. 58th ed. Thomson PDR. Montvale, NJ 2004., p. 1130

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
33
PharmaCompass offers a list of Benzocaine API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Benzocaine manufacturer or Benzocaine supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Benzocaine manufacturer or Benzocaine supplier.
PharmaCompass also assists you with knowing the Benzocaine API Price utilized in the formulation of products. Benzocaine API Price is not always fixed or binding as the Benzocaine Price is obtained through a variety of data sources. The Benzocaine Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A 06952_FLUKA manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of 06952_FLUKA, including repackagers and relabelers. The FDA regulates 06952_FLUKA manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. 06952_FLUKA API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of 06952_FLUKA manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A 06952_FLUKA supplier is an individual or a company that provides 06952_FLUKA active pharmaceutical ingredient (API) or 06952_FLUKA finished formulations upon request. The 06952_FLUKA suppliers may include 06952_FLUKA API manufacturers, exporters, distributors and traders.
click here to find a list of 06952_FLUKA suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A 06952_FLUKA DMF (Drug Master File) is a document detailing the whole manufacturing process of 06952_FLUKA active pharmaceutical ingredient (API) in detail. Different forms of 06952_FLUKA DMFs exist exist since differing nations have different regulations, such as 06952_FLUKA USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A 06952_FLUKA DMF submitted to regulatory agencies in the US is known as a USDMF. 06952_FLUKA USDMF includes data on 06952_FLUKA's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The 06952_FLUKA USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of 06952_FLUKA suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The 06952_FLUKA Drug Master File in Japan (06952_FLUKA JDMF) empowers 06952_FLUKA API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the 06952_FLUKA JDMF during the approval evaluation for pharmaceutical products. At the time of 06952_FLUKA JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of 06952_FLUKA suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a 06952_FLUKA Drug Master File in Korea (06952_FLUKA KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of 06952_FLUKA. The MFDS reviews the 06952_FLUKA KDMF as part of the drug registration process and uses the information provided in the 06952_FLUKA KDMF to evaluate the safety and efficacy of the drug.
After submitting a 06952_FLUKA KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their 06952_FLUKA API can apply through the Korea Drug Master File (KDMF).
click here to find a list of 06952_FLUKA suppliers with KDMF on PharmaCompass.
A 06952_FLUKA CEP of the European Pharmacopoeia monograph is often referred to as a 06952_FLUKA Certificate of Suitability (COS). The purpose of a 06952_FLUKA CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of 06952_FLUKA EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of 06952_FLUKA to their clients by showing that a 06952_FLUKA CEP has been issued for it. The manufacturer submits a 06952_FLUKA CEP (COS) as part of the market authorization procedure, and it takes on the role of a 06952_FLUKA CEP holder for the record. Additionally, the data presented in the 06952_FLUKA CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the 06952_FLUKA DMF.
A 06952_FLUKA CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. 06952_FLUKA CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of 06952_FLUKA suppliers with CEP (COS) on PharmaCompass.
A 06952_FLUKA written confirmation (06952_FLUKA WC) is an official document issued by a regulatory agency to a 06952_FLUKA manufacturer, verifying that the manufacturing facility of a 06952_FLUKA active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting 06952_FLUKA APIs or 06952_FLUKA finished pharmaceutical products to another nation, regulatory agencies frequently require a 06952_FLUKA WC (written confirmation) as part of the regulatory process.
click here to find a list of 06952_FLUKA suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing 06952_FLUKA as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for 06952_FLUKA API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture 06952_FLUKA as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain 06952_FLUKA and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a 06952_FLUKA NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of 06952_FLUKA suppliers with NDC on PharmaCompass.
06952_FLUKA Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of 06952_FLUKA GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right 06952_FLUKA GMP manufacturer or 06952_FLUKA GMP API supplier for your needs.
A 06952_FLUKA CoA (Certificate of Analysis) is a formal document that attests to 06952_FLUKA's compliance with 06952_FLUKA specifications and serves as a tool for batch-level quality control.
06952_FLUKA CoA mostly includes findings from lab analyses of a specific batch. For each 06952_FLUKA CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
06952_FLUKA may be tested according to a variety of international standards, such as European Pharmacopoeia (06952_FLUKA EP), 06952_FLUKA JP (Japanese Pharmacopeia) and the US Pharmacopoeia (06952_FLUKA USP).

