



API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
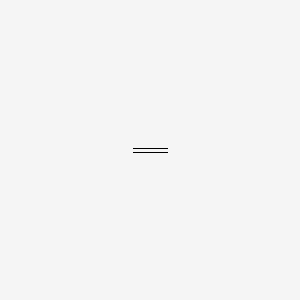

1. Ethene
1. Ethene
2. Acetene
3. Elayl
4. Olefiant Gas
5. Polyethylene
6. 74-85-1
7. 9002-88-4
8. Athylen
9. Etileno
10. Liquid Ethylene
11. Bicarburretted Hydrogen
12. Caswell No. 436
13. Ethylene, Pure
14. Aethylen
15. C2h4
16. Hsdb 168
17. Aethen
18. Liquid Ethyene
19. Epa Pesticide Chemical Code 041901
20. Ethylene (8ci)
21. Ethene (9ci)
22. Ch2=ch2
23. H2c=ch2
24. Polyethylene As Med Mol. Wt.
25. Chebi:18153
26. 91gw059kn7
27. Mfcd00084423
28. Un 1038
29. Un 1962
30. Plastipore
31. Athylen [german]
32. Polyethylene As
33. Ethylene [nf]
34. Ldpe
35. Einecs 200-815-3
36. Un1038
37. Un1962
38. Ethyleneradical
39. Ethylene Latex
40. Unii-91gw059kn7
41. Ethylene, Compressed
42. Ethene, 9ci
43. Ethylene-cmpd
44. Ethylene [hsdb]
45. Ethylene [iarc]
46. Ethylene [ii]
47. Ethylene [mi]
48. Ethylene, 99.99%
49. Ec 200-815-3
50. Ethylene, >=99.5%
51. Ethylene, >=99.9%
52. Ethylene, Compressed [un1962] [flammable Gas]
53. Carboneum Hydrogenisatum
54. Chembl117822
55. Ethylene, Purum, >=99.9%
56. Dtxsid1026378
57. Polyethylene Granules, High Density
58. Cmc_13849
59. Akos015915514
60. Carboneum Hydrogenisatum [hpus]
61. Polyethylene, Low Density, 500 Micron
62. Polyethylene, Low Density, 1000 Micron
63. Ethylene, Messer(r) Cangas, 99.98%
64. Ethylene, Puriss., >=99.95% (gc)
65. Polyethylene, Low Density, <=400 Micron
66. Ft-0626287
67. C06547
68. C19503
69. R-1150
70. Ethylene, Refrigerated Liquid (cryogenic Liquid)
71. Ethylene, Compressed [un1962] [flammable Gas]
72. Q151313
73. Polyethylene Rod, Low Density, 16mm (0.63in) Dia
74. Polyethylene Rod, Low Density, 19mm (0.75in) Dia
75. Polyethylene, Uhmw, >150 Micron, Mw 3-6 Million
76. Polyethylene Rod, High Density, 19mm (0.75in) Dia
77. Polyethylene Rod, Low Density, 12.7mm (0.5in) Dia
78. Q27286698
79. Polyethylene Rod, High Density, 6.35mm (0.25in) Dia
80. Polyethylene Rod, Low Density, 6.35mm (0.25in) Dia
81. Polyethylene Sheet, Low Density, 12.7mm (0.5in) Thick
82. Polyethylene Sheet, High Density, 1.6mm (0.063in) Thick
83. Polyethylene Sheet, High Density, 12.7mm (0.5in) Thick
84. Polyethylene Sheet, High Density, 6.35mm (0.25in) Thick
85. Polyethylene Sheet, Low Density, 1.6mm (0.063in) Thick
86. Polyethylene Sheet, Low Density, 3.18mm (0.125in) Thick
87. Polyethylene Sheet, Low Density, 6.35mm (0.25in) Thick
88. Polyethylene Sheet, High Density, 3.18mm (0.125in) Thick
89. Ethylene, Refrigerated Liquid (cryogenic Liquid) [un1038] [flammable Gas]
| Molecular Weight | 28.05 g/mol |
|---|---|
| Molecular Formula | C2H4 |
| XLogP3 | 1.2 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 0 |
| Rotatable Bond Count | 0 |
| Exact Mass | 28.0313001276 g/mol |
| Monoisotopic Mass | 28.0313001276 g/mol |
| Topological Polar Surface Area | 0 Ų |
| Heavy Atom Count | 2 |
| Formal Charge | 0 |
| Complexity | 0 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
/SRP: Former use/ ... For analgesia, few inhalations of 25-35% mixture with oxygen. For induction of anesthesia, 80-90% concentration of ethylene with 10-20% oxygen ... . However, 90% concentration ... should be given for no longer than 2-3 min. Patients usually can be maintained on mixture of 80% ethylene and 20% oxygen. If satisfactory anesthesia cannot be attained with ethylene, gas must be supplemented with barbiturate, strong analgesic, or other anesthetic vapor (eg, ether, halothane).
American Medical Association, Council on Drugs. AMA Drug Evaluations. 2nd ed. Acton, Mass.: Publishing Sciences Group, Inc., 1973., p. 227
It has disadvantage of providing inadequate muscle relaxation. Concentrations sufficiently high to induce hypoxia must be employed and the gas-oxygen mixtures are explosive; fatal accidents have occurred during ethylene anesthesia. Consequently, its use has declined markedly in recent years.
Osol, A. (ed.). Remington's Pharmaceutical Sciences. 16th ed. Easton, Pennsylvania: Mack Publishing Co., 1980., p. 987
Because of the high concentration of ethylene ... Required to produce and maintain anesthesia, cyanosis is an unavoidable accompaniment of ... /its former/ use.
Thienes, C., and T.J. Haley. Clinical Toxicology. 5th ed. Philadelphia: Lea and Febiger, 1972., p. 53
Plant Growth Regulators
Any of the hormones produced naturally in plants and active in controlling growth and other functions. There are three primary classes: auxins, cytokinins, and gibberellins. (See all compounds classified as Plant Growth Regulators.)
When equilibrium is reached, the rate of transfer of gas molecules from the alveolar space to blood equals the rate of removal by blood from the alveolar space. For example, ... ethylene has a low (0.14) blood/gas phase solubility ratio. For a substance with a low solubility ratio such as ethylene, only a small percentage of the total gas is removed by blood during each circulation because blood is soon saturated with the gas.
Bingham, E.; Cohrssen, B.; Powell, C.H.; Patty's Toxicology Volumes 1-9 5th ed. John Wiley & Sons. New York, N.Y. (2001)., p. 116
Ethylene has been determined in expired air of 2/8 human subjects at rate of 0.91 and 120 ug/hr.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V19 161 (1979)
... Excreted in urine ...
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V19 163 (1979)
The inhalation pharmacokinetics of ethylene have been investigated in human volunteers at atmospheric concentrations of up to 50 ppm (157.5 mg/cu m) by gas uptake in a closed spirometer system. The uptake, exhalation and metabolism of ethylene can be described by first-order kinetics. Uptake of ethylene into the body is low. Clearance due to uptake, which reflects the transfer rate of ethylene from the atmosphere into the body, was 25 L/hr for a man of 70 kg. This value represents only 5.6% of the experimentally obtained alveolar ventilation rate of 150 L/hr. The majority (94.4%) of ethylene inhaled into the lungs is exhaled again without becoming systemically available via the blood stream. Maximal accumulation of ethylene in the same man, determined as the thermodynamic partition coefficient whole body:air was 0.53. The concentration ratio at steady state was even smaller (0.33), owing to metabolic elimination. Clearance due to metabolism, in relation to the concentration in the atmosphere, was calculated to be 9.3 L/hr for a man of 70 kg. This indicates that at steady state about 36% of systemically available ethylene is eliminated metabolically and 64% is eliminated by exhalation as the unchanged substance, as can be calculated from the values of clearance of uptake and of clearance of metabolism. The biological half-life of ethylene was 0.65 hr. The alveolar retention of ethylene at steady state was calculated to be 2%. From theoretical considerations of the lung uptake of gases and vapors, it can be deduced that the low uptake rate of ethylene is due to its low solubility in blood: Ostwald's solubility coefficient for human blood at 37 C, 0.15.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V60 53 (1994)
For more Absorption, Distribution and Excretion (Complete) data for Ethylene (8 total), please visit the HSDB record page.
Rat liver microsomal monooxygenases transform ethylene to oxirane. ...
PMID:6318396 Schmiedel G et al; Toxicol Lett 19 (3): 293-7 (1983)
Male CBA mice exposed to air containing 19.6 mg/cu m ... (14)C-labeled ethylene metabolized ethylene to ethylene oxide, which binds to cellular proteins.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V19 163 (1979)
Four male CBA mice (average body weight, 31 g) were exposed together for 1 hr in a closed glass chamber (5.6 L) to (14)C-ethylene (22 mCi/mmol) in air at 17 ppm x hr (22.3 (mg/cu m) x hr, equivalent to about 1 mg/kg bw). Blood and organs from two mice were pooled 4 hr after the end of exposure. Radioactivity was about the same in kidney (0.16 uCi/g wet weight) and liver (0.14 uCi/g) but lower in testis (0.035 uCi/g), brain (0.02 uCi/g) and Hb (0.0094 uCi/g Hb). Urine was collected from the two other mice during 48 hr, and blood was collected after 21 days. 5-(2-Hydroxyethyl)cysteine was identified as a metabolite of ethylene in urine (3% of (14)C in urine) by thin-layer chromatography. The radioactivity in Hb was 0.011 uCi/g Hb. These data, together with those on specific hydroxyethyl derivatives at amino acid residues of Hb, indicated that ethylene was metabolized to ethylene oxide.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V60 55 (1994)
Experiments proved ethylene to be converted in certain species, notably mice and rats, into the carcinogenic and mutagenic ethylene oxide. Carcinogenic effect of ethylene of endogenous origin is suggested. Whether such an effect is possible with oral administration of ethylene is not clear.
Sheftel, V.O.; Indirect Food Additives and Polymers. Migration and Toxicology. Lewis Publishers, Boca Raton, FL. 2000., p. 83
The biological half-life of ethylene /in humans/ was 0.65 hr.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V60 53 (1994)
Ethylene interferes with the activities of plant hormones causing growth retardation.
Waldbott GL; Health Eff Environ Pollut p.47 (1973)


