



API Suppliers

US DMFs Filed

CEP/COS Certifications

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0

USA (Orange Book)
0

Europe

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
USP
0
JP
0
Other Listed Suppliers
0
0
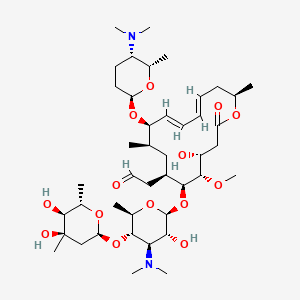

1. Demycarosylturimycin H
2. Spiramycin I
1. Foromacidine A
2. Spiramycin I
3. Spiramycin 1
4. Demycarosylturimycin H
5. Spiramycin
6. Foromacidin A
7. Foromacidin
8. 24916-50-5
9. Rp 5337
10. 8025-81-8
11. Nsc55926
12. Rovamycinetrade Mark
13. Nsc64393
14. Ic 5902
15. Chebi:85260
16. Db06145
17. J-015730
18. [(4r,5s,6s,7r,9r,10r,11e,13e,16r)-6-{[(2s,3r,4r,5s,6r)-5-{[(2s,4r,5s,6s)-4,5-dihydroxy-4,6-dimethyloxan-2-yl]oxy}-4-(dimethylamino)-3-hydroxy-6-methyloxan-2-yl]oxy}-10-{[(2r,5s,6s)-5-(dimethylamino)-6-methyloxan-2-yl]oxy}-4-hydroxy-5-methoxy-9,16-dimethyl-2-oxo-1-oxacyclohexadeca-11,13-dien-7-yl]acetaldehyde
19. 2-[(4r,5s,6s,7r,9r,10r,11e,13e,16r)-6-[(2s,3r,4r,5s,6r)-5-[(2s,4r,5s,6s)-4,5-dihydroxy-4,6-dimethyl-tetrahydropyran-2-yl]oxy-4-(dimethylamino)-3-hydroxy-6-methyl-tetrahydropyran-2-yl]oxy-10-[(2r,5s,6s)-5-(dimethylamino)-6-methyl-tetrahydropyran-2-yl]oxy-4-hydroxy-5-methoxy-9,16-dimethyl-2-oxo-1-oxacyclohexadeca-11,13-dien-7-yl]acetaldehyde
| Molecular Weight | 843.1 g/mol |
|---|---|
| Molecular Formula | C43H74N2O14 |
| XLogP3 | 2.1 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 16 |
| Rotatable Bond Count | 11 |
| Exact Mass | 842.51400504 g/mol |
| Monoisotopic Mass | 842.51400504 g/mol |
| Topological Polar Surface Area | 195 Ų |
| Heavy Atom Count | 59 |
| Formal Charge | 0 |
| Complexity | 1370 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 19 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 2 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anti-Bacterial Agents; Coccidiostats
National Library of Medicine's Medical Subject Headings. Spiramycin. Online file (MeSH, 2015). Available from, as of August 19, 2015: https://www.nlm.nih.gov/mesh/2014/mesh_browser/MBrowser.html
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Spiramycin is included in the database.
NIH/NLM; ClinicalTrials.Gov. Available from, as of October 1, 2015: https://clinicaltrials.gov/search/intervention=spiramycin
MEDICATION (VET): Spiramycin is a macrolide antibiotic used for the treatment and control of a number of bacterial and mycoplasmal infections in animals. It is available as a spiramycin embonate for use in animal feed, and as the adipate, a more soluble form, for administration by other routes.
WHO/FAO; Joint Meeting on Food Additives; Toxicological Evaluation of Certain Veterinary Drug Residues in Food, WHO Food Additive Series 29: Spiramycin (1991). Available from, as of October 27, 2015: https://www.inchem.org/pages/jecfa.html
The aim of this study is to evaluate the efficacy of spiramycin in prevention of mother-to-child transmission of Toxoplasma gondii infection. Patients within first trimester of their pregnancy with Toxoplasma IgM positivity (>0.65 index, ELISA, VIDAS) and IgG positivity (>8 IU/ml), who had low IgG avidity (<0.50 index, ELISA, Architet) were considered as having acute toxoplasmosis. These patients who had amniocentesis at the 19th-21st week of pregnancy were examined for the detection of Toxoplasma DNA. Detailed ultrasonographic examinations performed between the 20th and 24th gestational weeks and the mothers and babies were followed for at least one year. ut of 61 patients, 55 (90.2%) had received Spy prophylaxis while 6 (9.8%) cases refused Spy prophylaxis. Toxoplasma PCR test was found to be positive in amniotic fluid of 4 (6.6%) patients obtained by amniocentesis at the 19th-21st week of pregnancy. All four of these patients had refused Spy prophylaxis had positive Toxoplasma PCR in amniotic fluid (p < 0.01). Our results seem to encourage the use of spiramycin in women with toxoplasmosis during pregnancy.
PMID:26365472 Avci ME et al; J Matern Fetal Neonatal Med 12: 1-4 (2015)
Spiramycin is a macrolide antibacterial that is used similarly to erythromycin in the treatment of susceptible bacterial infections. It has also been used in the protozoal infections cryptosporidiosis and toxoplasmosis.
SWEETMAN, S.C. (ed.) Martindale-The Complete Drug Reference. 36th ed. London: The Pharmaceutical Press, 2009., p. 333
The most frequent adverse effects are gastrointestinal disturbances. Transient parethesia has been reported during parenteral use.
SWEETMAN, S.C. (ed.) Martindale-The Complete Drug Reference. 36th ed. London: The Pharmaceutical Press, 2009., p. 333
Spiramycin, a 16-membered lactone ring macrolide, has been in clinical use for the past 15 years with little serious associated toxicity. GI disturbance has usually been mild & no changes in GI motility have been noted either experimentally or in humans, in contrast to other macrolides, such as erythromycin. Allergic reactions have been uncommon & mainly restricted to transient skin eruptions. Although liver injury is a possible complication of most macrolide treatments, no conclusive evidence for spiramycin-induced hepatitis is currently available, and, again in contrast to most other macrolides, the lack of drug interactions with spiramycin has been clearly established in biochemical, pharmacokinetic & clinical studies.
Descotes J, et al; J Antimicrob Chemother 22 (Suppl B): 207-210 (1988)
... Allergic drug reactions to macrolides are extremely rare & there is little information in the literature concerning relevant diagnostic tests. ... Twenty-one patients were recently seen for assumed allergies (principally urticaria) to diverse macrolides. Skin tests (prick & intradermal tests) were performed with injectable forms of spiramycin & erythromycin. Seventeen out of 21 patients were provoked under strict hospital surveillance. ... Only 3 patients had a positive provocation test & were thus truly allergic (to spiromycin). They had positive skin tests to both macrolides tested. ... Most hypersensitivity reactions to macrolides are therefore diagnosed with provocation tests.
PMID:10719443 Demoly P, et al; Presse Med 29 (6): 294-298 (2000)
We recently reported two cases of QT interval prolongation & cardiac arrest in newborns receiving antibiotic therapy with spiramycin, a macrolide agent extensively used for toxoplasmosis prophylaxis. In this study we assessed the effects of this drug on ventricular repolarization & on the potential risk of lethal arrhythmias in 8 newborn infants in whom toxoplasmosis prophylaxis after birth was necessary. Electrocardiograms (ECGs) & echocardiograms were recorded during spiramycin therapy (350,000 i.u./kg/ day) & after its withdrawal. In a control group of 8 healthy newborns matched for age & sex, no differences were found between 2 ECGs analogously recorded. The QT interval corrected for heart rate (QTc) was longer during spiramycin therapy than after drug withdrawal (448 +/- 32 msec vs 412 +/- 10 msec, +9%, p=0.021). QTc dispersion, expressed as the difference between the longest & the shortest value in 12 different leads (QTcmax-min), was also higher during spiramycin therapy (60 +/- 32 msec vs 34 +/- 8 msec, +76%, p=0.021), mainly because of a major lengthening of the longest QTc (QTcmax). QTc & QTc dispersion were markedly increased in the 2 newborns who experienced cardiac arrest after beginning treatment compared with the 6 neonates who had no drug-induced symptoms. During therapy 7 of 8 newborns had a rare abnormality in the thickening of the left ventricular posterior wall similar to that observed in patients with congenital long QT syndrome. This abnormality disappeared after drug withdrawal. Thus antibiotic therapy with spiramycin in the neonatal period may induce QT interval prolongation & incr QT dispersion. When this effect on ventricular repolarization is more marked, it may favor the occurrence of torsades des pointes & lead to cardiac arrest.
PMID:9006298 Stramba-Badiale M, et al; Am Heart J 133 (1): 108-111 (1997)
Macrolide antibiotic for treatment of various infections.
The absolute bioavailability of oral spiramycin is generally within the range of 30 to 40%. After a 1 g oral dose, the maximum serum drug concentration was found to be within the range 0.4 to 1.4 mg/L.
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01F - Macrolides, lincosamides and streptogramins
J01FA - Macrolides
J01FA02 - Spiramycin
Absorption
The extent of absorption of Spiramycin was shown to be incomplete. Oral bioavailability ranges from 30-39%. Spiramycin has slower rate of absorption than Erythromycin. It has a high pKa (7.9) which could be a result of high degree of ionization in acidic medium of the stomach.
Route of Elimination
Fecal-biliary route is the primary route of elimination. The secondary route is renal-urinary route.
Volume of Distribution
The tissue distribution of spiramycin is extensive. The volume of distribution is in excess of 300 L, and concentrations achieved in bone, muscle, respiratory tract and saliva exceed those found in serum. Spiramycin showed high concentrations in tissues such as: lungs, bronchi, tonsils, and sinuses.
Clearance
80% of the administered dose excreted in the bile, which makes the fecal-biliary route is the most important route of elimination. Enterohepatic recycling could also occur. Only 4 to 14% of an administered dose is eliminated through renal-urinary excretion route.
Spiramycin is well absorbed in humans after oral administration. Oral administration of 15-30 mg/kg bw to healthy young male adults resulted in peak plasma levels in 3-4 hours and plasma concentrations of 0.96-1.65 mg/l. After intravenous dosing (7.25 mg/kg b.w.) a large volume of distribution (Vdss 5.6 l/kg) was observed indicating extensive tissue distribution. Biotransformation did not appear to be important. Biliary excretion was the main route of excretion; only 7-20% of an oral dose was excreted in the urine. Spiramycin is known to achieve high tissue:serum concentrations in pulmonary and prostatic tissues, and in skin.
WHO/FAO; Joint Meeting on Food Additives; Toxicological Evaluation of Certain Veterinary Drug Residues in Food, WHO Food Additive Series 29: Spiramycin (1991). Available from, as of October 27, 2015: https://www.inchem.org/pages/jecfa.html
Spiramycin crosses the placenta to the fetus. Concns of the antibiotic in maternal serum, cord blood, & the placenta after a dosage regimen of 2 g/day were 1.19 ug/ml, 0.63 ug/ml, & 2.75 ug/ml, respectively. When the maternal dose was increased to 3 g/day, the levels were 1.69 ug/ml, 0.78 ug/ml, & 6.2 ug/ml, respectively. Based on these results, the cord:maternal serum ratio is approx 0.5. Moreover, at these doses, spiramycin is concentrated in the placenta with levels approx 2-4 times those in the maternal serum. ... Spiramycin is excreted into breast milk. Nursing infants of mothers receiving 1.5 g/day for 3 days had spiramycin serum concns of 20 ug/ml. This concn was bacteriostatic.
Briggs, G.G, R.K. Freeman, S.J. Yaffe. A Reference Guide to Fetal and Neonatal Risk. Drugs in Pregnancy and Lactation. 4th ed. Baltimore, MD: Williams & Wilkins 1994., p. 788
/MILK/ Spiramycin is a macrolide antibiotic that is active against most of the microorganisms isolated from the milk of mastitic cows. This work investigated the disposition of spiramycin in plasma & milk after iv, intramuscular & subcutaneous admin. Twelve healthy cows were given a single injection of spiramycin at a dose of 30,000 IU/kg by each route. Plasma & milk were collected post injection. Spiramycin concn in the plasma was determined by a high performance liquid chromatography method, & in the milk by a microbiological method. The mean residence time after iv admin was significantly longer (P<0.01) in the milk (20.7 +/- 2.7 h) than in plasma (4.0 +/- 1.6 h). An average milk-to-plasma ratio of 36.5 +/- 15 was calculated from the area concn-time curves. Several pharmacokinetic parameters were examined to determine the bioequivalence of the two extravascular routes. The dose fraction adsorbed after intramuscular or subcutaneous admin was almost 100% & was bioequivalent for the extravascular routes, but the rates of absorption, the max concns & the time to obtain them differed significantly between the two routes. Spiramycin quantities excreted in milk did not differ between the two extravascular routes but the latter were not bioequivalent for max concn in the milk. However, the two routes were bio-equivalent for the duration of time the milk concn exceeded the minimal inhibitory concn (MIC) of various pathogens causing infections in the mammary gland.
PMID:1573706 Sanders P, et al; J Vet Pharmacol Ther 15 (1): 53-61 (1992)
Plasma protein binding ranges from 10 to 25%. An oral dose of 6 million units produces peak blood concentrations of 3.3 ug/mL after 1.5 to 3 hours; the half life is about 5 to 8 hours. High tissue concentrations are achieved and persist long after the plasma concentration has fallen to low levels.
SWEETMAN, S.C. (ed.) Martindale-The Complete Drug Reference. 36th ed. London: The Pharmaceutical Press, 2009., p. 333
For more Absorption, Distribution and Excretion (Complete) data for SPIRAMYCIN (13 total), please visit the HSDB record page.
Spiramycin is less metabolised than some of the other macrolides. Metabolism has not been well studied. It is mainly done in the liver to the active metabolites.
In cattle, the metabolite neospiramycin, the demycarosyl derivative, is formed. Concentrations of neospiramycin in muscle and kidney were marginally higher than those of spiramycin 14-28 days after dosing; in muscle, levels of neospiramycin and spiramycin were approximately equal.
WHO/FAO; Joint Meeting on Food Additives; Toxicological Evaluation of Certain Veterinary Drug Residues in Food, WHO Food Additive Series 29: Spiramycin (1991). Available from, as of October 27, 2015: https://www.inchem.org/pages/jecfa.html
Spiramycin is metabolized in the liver to active metabolites; substantial amounts are excreted in the bile and about 10% in the urine.
SWEETMAN, S.C. (ed.) Martindale-The Complete Drug Reference. 36th ed. London: The Pharmaceutical Press, 2009., p. 333
Intravenous: Young persons (18 to 32 years of age): Approximately 4.5 to 6.2 hours. Elderly persons (73 to 85 years of age): Approximately 9.8 to 13.5 hours. Oral: 5.5-8 hours, Rectal in children: 8 hours
An oral dose of 6 million units produces ... /a/ half life is about 5 to 8 hours.
SWEETMAN, S.C. (ed.) Martindale-The Complete Drug Reference. 36th ed. London: The Pharmaceutical Press, 2009., p. 333
The mechanism of action of macrolides has been a matter of controversy for some time. Spiramycin, a 16-membered macrolide, inhibits translocation by binding to bacterial 50S ribosomal subunits with an apparent 1 : 1 stoichiometry. This antibiotic is a potent inhibitor of the binding to the ribosome of both donor and acceptor substrates. The primary mechanism of action is done by stimulation of dissociation of peptidyl-tRNA from ribosomes during translocation.I


