
Synopsis
Synopsis
0
USDMF
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Gas, Laughing
2. Laughing Gas
3. Nitrogen Protoxide
4. Oxide, Nitrous
1. Dinitrogen Oxide
2. Laughing Gas
3. Dinitrogen Monoxide
4. Factitious Air
5. Nitrogen Protoxide
6. Nitrogen Oxide (n2o)
7. 10024-97-2
8. Hyponitrous Acid Anhydride
9. Nitrogen Hypoxide
10. Stickdioxyd
11. Oxyde Nitreux
12. Oxido Nitroso
13. Nitrous Oxide [jan]
14. Protoxyde D'azote
15. Nitrous Oxide, Compressed
16. Lachgas
17. Gaz Hilarant
18. Diazyne 1-oxide
19. Distickstoffmonoxid
20. Fema No. 2779
21. Stickstoff(i)-oxid
22. Nitrous Oxide, Refrigerated Liquid
23. Nitrogenium Oxydulatum
24. Nitrous Oxide (tn)
25. N2o
26. Nitrogenum Oxygenatum
27. Nitrous Oxide [anaesthetics, Volatile]
28. Protoxide Of Nitrogen
29. Oxidodinitrogen(n--n)
30. Nitrogen Oxide (n(sub 2)o)
31. Ins No.942
32. Chebi:17045
33. Ins-942
34. K50xqu1029
35. E942
36. Stickdioxyd [german]
37. E-942
38. Oxide, Nitrous
39. Gas, Laughing
40. Oxido Nitroso [spanish]
41. Protoxyde D'azote [french]
42. Nno
43. Ccris 1225
44. Hsdb 504
45. Nitrous-oxide
46. Einecs 233-032-0
47. Un1070
48. Un2201
49. Nitrous Oxide [usp:jan]
50. Nitrous Oxide (jp15/usp)
51. Nitrious Oxide
52. Unii-k50xqu1029
53. Nitrous Oxide [un1070] [nonflammable Gas]
54. Dinitrogenii Oxidum
55. Diazyne 1-oxide #
56. 1,2-diazaethyne1-oxide
57. Ec 233-032-0
58. Nitrous Oxide [mi]
59. Nitrous Oxide [fcc]
60. Nitrous Oxide, Jan, Usan
61. Nitrous Oxide [fhfi]
62. Nitrous Oxide [hsdb]
63. Nitrous Oxide [inci]
64. Nitrous Oxide (jp17/usp)
65. Nitrous Oxide [vandf]
66. Nitrous Oxide [mart.]
67. Nitrous Oxide [who-dd]
68. Chembl1234579
69. Dtxsid8021066
70. Fema 2779
71. Dinitrogen Oxide [who-ip]
72. Nitrous Oxide [green Book]
73. Nitrous Oxide [ep Impurity]
74. Nitrous Oxide [ep Monograph]
75. R-744a
76. Nitrous Oxide [usp Monograph]
77. Akos015903682
78. Db06690
79. Un 1070
80. Un 2201
81. Dinitrogenii Oxidum [who-ip Latin]
82. Nitrous Oxide [un1070] [nonflammable Gas]
83. C00887
84. D00102
85. Q905750
86. Nitrous Oxide, Refrigerated Liquid [un2201] [nonflammable Gas]
| Molecular Weight | 44.013 g/mol |
|---|---|
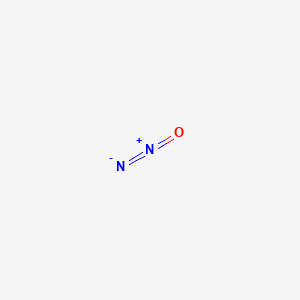
| Molecular Formula | N2O |
| XLogP3 | 0.5 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Exact Mass | 44.001062628 g/mol |
| Monoisotopic Mass | 44.001062628 g/mol |
| Topological Polar Surface Area | 19.1 Ų |
| Heavy Atom Count | 3 |
| Formal Charge | 0 |
| Complexity | 25.8 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Analgesics, Non-Narcotic; Anesthetics, Inhalation
National Library of Medicine's Medical Subject Headings online file (MeSH, 2016)
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Nitrous oxide is included in the database.
NIH/NLM; ClinicalTrials.Gov. Available from, as of March 17, 2016: https://clinicaltrials.gov/ct2/results?term=nitrous+oxide&Search=Search
Although 50% nitrous oxide (N(2)O) and oxygen is a widely used treatment, its efficacy had never been evaluated in the prehospital setting. The objective of this study was to demonstrate the efficacy of premixed N(2)O and oxygen in patients with out-of-hospital moderate traumatic acute pain. This prospective, randomized, multicenter, double-blind trial enrolled patients with acute moderate pain (numeric rating scale [NRS] score between 4 and 6 out of 10) caused by trauma. Patients were assigned to receive either 50/50 N(2)O and oxygen 9 L/min (N(2)O group) or medical air (MA) 9 L/min (MA group), in ambulances from two nurse-staffed fire department centers. After the first 15 minutes, every patient received N(2)O and oxygen. The primary endpoint was pain relief at 15 minutes (T15), defined as a NRS
PMID:23406077 Ducasse JL et al; Acad Emerg Med 20 (2): 178-84 (2013)
The aim of this study was to investigate the effects of N2O-O2 mixture (Inspired O2 30%) on middle ear pressure (MEP) in children compared with the effects of an air-oxygen mixture (Inspired O2 50%). The study included thirty child patients who underwent general anesthesia for different reasons, with the exception of ENT problems and ear interventions. They were randomly divided into two groups. Group 1 (15 children: 10 male and 5 female) received a N2O-O2 mixture (Inspired O2 30%); and group 2 (15 children: 10 male and 5 female) were given an air-oxygen mixture (Inspired O2 50%). MEP was measured using a portable impedance analyzer before the operation (PreO),10 minutes after intubation (10AEn), 30 minutes after intubation (30AEn), 10 minutes before extubation (10BEx), 15 minutes after the operation (PO15), 30 minutes after the operation (PO30), 1 hour after the operation (PO1h) and 6 hours after the operation (PO6h). The pressure and compliance values were the same in groups 1 and 2. The pressure-time graphs for the two groups were different: in Group 2, MEP rose quickly at 10AEn and positive pressure values were seen in the middle ear. MEP then fell rapidly until the end of the surgery and lower and negative pressures (Mean -50 daPa) were observed at PO6h. In Group 1, MEP was elevated at 10AEn and positive pressure was found (but not as high as in Group 2). MEP then fell more slowly. In other words, positive pressure in the middle ear persisted longer and the middle ear was subjected to positive pressure and nitrogen over a longer period. Separate analyses were made in Groups 1 and 2 of pressure differences and of compliance values at eight measurement points using the Friedman test. Differences in pressure values were found to be statistically significant in both Group 1 ... and Group 2 ... . In Group 1, all the 10AEn and 30AEn values were significantly higher than the PreO, PO30, PO1h and PO6h values. The 10BEx value was significantly higher than the PreO and PO1h values. The PO15 value was significantly higher than the PreO value. In Group 2, the PO6h value was significantly lower than the 10BEx, 10AEn and 30AEn values. The PO1h value was significantly lower than the 30AEn values. The MEP values increased in Group 1 in younger and taller children and in children receiving anesthesia for shorter periods. MEP values increased in Group 2 in younger and taller children, and in heavier children. MEP values fell with the length of anesthesia. In brief anesthesia, nitrogen was not removed from the middle ear quickly in Group 1: middle ear pressure values were higher. The nitrous oxide remained in the middle ear longer and so the possibility of ear toxicity may increase. In Group 2, 50% O2 was rapidly absorbed and removed from the middle ear and so middle ear pressure was not as high. It may be concluded that air-oxygen mixture (Inspired O2 50%) anesthesia should be recommended as being more reliable in tympanoplasties and other middle ear interventions than a N2O-O2 mixture (Inspired O2 30%).
PMID:23909121 Apan A et al; B-ENT 9 (2): 141-50 (2013)
For more Therapeutic Uses (Complete) data for Nitrous oxide (17 total), please visit the HSDB record page.
A 65 year-old man with no medical history, presented problems walking and memory loss 16 days after surgery for femoral prosthesis. Neurological examination revealed paraplegia with syndrome of combined degeneration of the spinal cord. The exploration of cognitive functions showed disorientation in time with memory disorders and disturbance of executive functioning. There was no apraxia, aphasia or agnosia. There were neither psychotic symptoms nor mood changes. ... Red blood count revealed an anemia with macrocytosis (MCV=120 3). Vitamin B12 rate was very low. Folate blood level was normal. Brain MRI showed moderate cerebral atrophy. Other investigations led to the diagnosis of Biermer's disease (fundic atrophy at biopsy with presence in the serum of antibodies to intrinsic factor). The diagnosis of neurological attack related to a vitamin B12 deficiency secondary to Biermer's disease was established, but the appearance of disorders in the post-operative period suggested the existence of an added factor. ... Anesthesia was maintained by nitrous oxide during two hours and the patient exhibited pre-operative anemia with macrocytosis. The hypothesis of decompensation of latent vitamin B12 deficiency by nitrous oxide was evoked. Replacement therapy by vitamin B12 induced real improvement of the cognitive impairment...
PMID:17457299 El Otmani H et al; Encephale 33 (1): 95-7 (2007)
... Two patients ... presented gait disorders after nitrous oxide anesthesia. Physical examination revealed arms and legs pyramidal syndrome and abnormal proprioception, consistent with subacute combined degeneration of the spinal cord. Serum vitamin B12 level was extremely low. The patients improved with parenteral treatment with hydroxycobalamin. CONCLUSIONS: ... Anesthesia-related exposure to nitrous oxide may induce neurologic disorders even in patients with no preliminary vitamin B12 deficiency.
PMID:17404524 Cohen A et al; Rev Neurol (Paris) 163 (3): 362-4 (2007)
... Several investigators have shown that prolonged administration of anesthetic drugs, including ketamine, isoflurane, nitrous oxide and midazolam, produced increased neurodegeneration in 7-day-old rat pups. The combination of the latter three drugs led to altered learning behavior in adulthood. Despite these unequivocal findings in rodents, similar changes cannot be reproduced in other species. Furthermore, withholding anesthesia during painful procedures in neonatal rats resulted in significant long-term aberrant responses to sensory stimulation and pain thresholds. SUMMARY: Taken together, these studies question the applicability of these data to the anesthetic management of the neonate. Further investigations in this area are needed before withholding anesthetics in the anesthetic management of pediatric surgical patients.
PMID:16534354 Soriano SG, Anand KJ; Curr Opin Anaesthesiol 18 (3): 293-7 (2005)
In a population of patients at risk for ischemic brain injury, nitrous oxide use had no overall beneficial or detrimental impact on neurologic or neuropsychological outcomes.
PMID:18362587 McGregor DG et al; Anesthesiology 108 (4): 568-79 (2008). Comment in: Anesthesiology 108 (4): 553-4 (2008)
For more Drug Warnings (Complete) data for Nitrous oxide (22 total), please visit the HSDB record page.
Lethal nitrous oxide blood level 350 mg/L, 35 mg/dL
Winek, C.L. Drug and Chemical Blood-Level Data 1985. Pittsburgh, PA: Allied Fischer Scientific, 1985.
Sedation
Analgesics, Non-Narcotic
A subclass of analgesic agents that typically do not bind to OPIOID RECEPTORS and are not addictive. Many non-narcotic analgesics are offered as NONPRESCRIPTION DRUGS. (See all compounds classified as Analgesics, Non-Narcotic.)
Anesthetics, Inhalation
Gases or volatile liquids that vary in the rate at which they induce anesthesia; potency; the degree of circulation, respiratory, or neuromuscular depression they produce; and analgesic effects. Inhalation anesthetics have advantages over intravenous agents in that the depth of anesthesia can be changed rapidly by altering the inhaled concentration. Because of their rapid elimination, any postoperative respiratory depression is of relatively short duration. (From AMA Drug Evaluations Annual, 1994, p173) (See all compounds classified as Anesthetics, Inhalation.)
N01AX13
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
N - Nervous system
N01 - Anesthetics
N01A - Anesthetics, general
N01AX - Other general anesthetics
N01AX13 - Nitrous oxide
The blood/gas partition coefficient is low and most of the inhaled nitrous oxide is rapidly eliminated through the lungs, though small amounts diffuse through the skin.
IPCS; Poisons Information Monograph PIM381: Nitrous oxide (February 1992). Available from, as of May 12, 2010: https://www.inchem.org/pages/pims.html
/Nitrous oxide/ is highly lipid soluble and rapidly absorbed and distributed throughout the body, particularly the vessel-rich regions, including the brain, heart, kidney, splanchnic circulation, and endocrine glands. The rate of nitrous oxide uptake during the first 1 or 2 min is about 1.0 L/min (at an inspired concentrations of 80%), with later uptake inversely proportional to the square root of time. /Nitrous oxide/ is relatively nonreactive and poorly soluble in blood. ... Little hepatic or renal metabolism is detectable in experimental animals, although intestinal bacteria can reduce small quantities of inhaled /nitrous oxide/ to nitrogen gas. Small amounts of inhaled /nitrous oxide/ are also eliminated through the skin and urine.
Bingham, E.; Cohrssen, B.; Powell, C.H.; Patty's Toxicology Volumes 1-9 5th ed. John Wiley & Sons. New York, N.Y. (2001)., p. V3 652-3
Placental transmission data: time to appear in fetus, 6 minutes; fetal/maternal concentration ratio, 0.6. /From table/
LaDu, B.N., H.G. Mandel, and E.L. Way. Fundamentals of Drug Metabolism and Disposition. Baltimore: Williams and Wilkins, 1971., p. 100
Concentration for surgical anesthesia: nitrous oxide-inhaled concn 80-85%; blood level 30-50 mg/100 mL; partition coefficients: blood/air 0.47; brain/blood 1.1; oil/blood 3; clearance rate of blood passing lung (alveolar tension) 63%.
Meyers, F.H., E. Jaetz, and A. Golfien. Review of Medical Pharmacology. Los Altos, California: Lange Medical Publications, 1972., p. 178
Nitrous oxide is almost completely eliminated by the lungs, with some minimal diffusion through the skin. Nitrous oxide is not biotransformed by enzymatic action in human tissue, and 99.9% of absorbed nitrous oxide is eliminated unchanged.
Brunton, L. Chabner, B, Knollman, B. Goodman and Gillman's The Pharmaceutical Basis of Therapeutics, Twelth Edition, McGraw Hill Medical, New York, NY. 2011, p. 546
Nitrous oxide is not biotransformed by enzymatic action in human tissue ... .
Brunton, L. Chabner, B, Knollman, B. Goodman and Gillman's The Pharmaceutical Basis of Therapeutics, Twelth Edition, McGraw Hill Medical, New York, NY. 2011, p. 546
Findings to date indicate that nitrous oxide induces opioid peptide release in the brain stem leading to the activation of descending noradrenergic neurones, which results in modulation of the nociceptive process in the spinal cord. Several receptoreffector mechanisms including dopamine receptors, 2 adrenoceptors, benzodiazepine receptors and -methyl- -aspartate (NMDA) receptors have been implicated although the relationship of one with the other is not known.
Nitrous oxide (N2O) gas is a widely used anesthetic adjunct in dentistry and medicine that is also commonly abused. Studies have shown that N2O alters the function of the N-methyl-d-aspartate (NMDA), GABAA, opioid, and serotonin receptors among others. However, the receptors systems underlying the abuse-related central nervous system effects of N2O are unclear. The present study explores the receptor systems responsible for producing the discriminative stimulus effects of N2O. B6SJLF1/J male mice trained to discriminate 10 minutes of exposure to 60% N2O + 40% oxygen versus 100% oxygen served as subjects. Both the high-affinity NMDA receptor channel blocker (+)-MK-801 maleate [(5S,10R)-(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine maleate] and the low-affinity blocker memantine partially mimicked the stimulus effects of N2O. Neither the competitive NMDA antagonist, CGS-19755 (cis-4-[phosphomethyl]-piperidine-2-carboxylic acid), nor the NMDA glycine-site antagonist, L701-324 [7-chloro-4-hydroxy-3-(3-phenoxy)phenyl-2(1H)-quinolinone], produced N2O-like stimulus effects. A range of GABAA agonists and positive modulators, including midazolam, pentobarbital, muscimol, and gaboxadol (4,5,6,7-tetrahydroisoxazolo[4,5-c]pyridine-3-ol), all failed to produce N2O-like stimulus effects. The mu-, kappa-, and delta-opioid agonists, as well as 5-hydroxytryptamine (serotonin) 1B/2C (5-HT1B/2C) and 5-HT1A agonists, also failed to produce N2O-like stimulus effects. Ethanol partially substituted for N2O. Both (+)-MK-801 and ethanol but not midazolam pretreatment also significantly enhanced the discriminative stimulus effects of N2O. Our results support the hypothesis that the discriminative stimulus effects of N2O are at least partially mediated by NMDA antagonist effects similar to those produced by channel blockers. However, as none of the drugs tested fully mimicked the stimulus effects of N2O, other mechanisms may also be involved.
PMID:25368340 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4279098 Richardson KJ, Shelton KL; J Pharmacol Exp Ther 352 (1): 156-65 (2015)
N2O interferes with vitamin B12 and folate metabolism. This impairs production of methionine (from homocysteine), used to form tetrahydrofolate and thymidine during DNA synthesis.
Myles PS et al; Anesthesiology 103: A681 (2005)
Nitrous oxide is 35 times more soluble than nitrogen. The gas exchanges with nitrogen and diffuses into hollow viscera and body spaces potentially containing air, such as pneumothorax, paranasal sinuses and pneumoperitoneum, or into the cerebral ventricles following pneumoencephalography. This expands the body of trapped air and increases the pressure within such closed spaces. When administration is discontinued, nitrous oxide is released into the alveoli, diluting the alveolar gases. A reduction in alveolar oxygen tension may result. This is referred to as diffusion anoxia. Because of the high concentration of nitrous oxide required to produce and maintain anesthesia, hypoxia is an unavoidable accompaniment to its use. During induction with high concentrations of nitrous oxide, the oxygen in the lungs is rapidly used up and the anoxia with increased respiratory effort causes rapid depletion of carbon dioxide in the tissues. Absence of carbon dioxide and depression of the medullary centers by the anesthetic quickly lead to respiratory failure, and rarely, the patient's cerebral function fails to recover from cerebral damage caused by the prolonged anoxia. The brain suffers anoxia from the very beginning of the administration of the gas, and not from just the moment of cessation of respiratory movements. Thus, the period of anoxia may be five minutes or more, sufficient to cause permanent brain damage in the susceptible individual. The arbitrary "safe period" of eight minutes may be too long for some patients.
IPCS; Poisons Information Monograph PIM381: Nitrous oxide (February 1992). Available from, as of February 26, 2016: https://www.inchem.org/pages/pims.html
Nitrous oxide induces inconsistent changes in the basal levels of the thalamic nuclei. The mechanism of analgesia is believed to involve a direct intraspinal anti-nociceptive action rather than depression of limbic function. In the brain stem, responses evoked by pain stimulation are depressed, although the extent of depression may be variable. Nitrous oxide in anesthetic doses increases cerebral blood flow and intracranial pressure.
IPCS; Poisons Information Monograph PIM381: Nitrous oxide (February 1992). Available from, as of February 26, 2016: https://www.inchem.org/pages/pims.html
For more Mechanism of Action (Complete) data for Nitrous oxide (7 total), please visit the HSDB record page.
Related Excipient Companies

Excipients by Applications
ABOUT THIS PAGE
20
PharmaCompass offers a list of Nitrous Oxide API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Nitrous Oxide manufacturer or Nitrous Oxide supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Nitrous Oxide manufacturer or Nitrous Oxide supplier.
PharmaCompass also assists you with knowing the Nitrous Oxide API Price utilized in the formulation of products. Nitrous Oxide API Price is not always fixed or binding as the Nitrous Oxide Price is obtained through a variety of data sources. The Nitrous Oxide Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A 00583_FLUKA manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of 00583_FLUKA, including repackagers and relabelers. The FDA regulates 00583_FLUKA manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. 00583_FLUKA API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A 00583_FLUKA supplier is an individual or a company that provides 00583_FLUKA active pharmaceutical ingredient (API) or 00583_FLUKA finished formulations upon request. The 00583_FLUKA suppliers may include 00583_FLUKA API manufacturers, exporters, distributors and traders.
click here to find a list of 00583_FLUKA suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A 00583_FLUKA CEP of the European Pharmacopoeia monograph is often referred to as a 00583_FLUKA Certificate of Suitability (COS). The purpose of a 00583_FLUKA CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of 00583_FLUKA EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of 00583_FLUKA to their clients by showing that a 00583_FLUKA CEP has been issued for it. The manufacturer submits a 00583_FLUKA CEP (COS) as part of the market authorization procedure, and it takes on the role of a 00583_FLUKA CEP holder for the record. Additionally, the data presented in the 00583_FLUKA CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the 00583_FLUKA DMF.
A 00583_FLUKA CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. 00583_FLUKA CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of 00583_FLUKA suppliers with CEP (COS) on PharmaCompass.
00583_FLUKA Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of 00583_FLUKA GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right 00583_FLUKA GMP manufacturer or 00583_FLUKA GMP API supplier for your needs.
A 00583_FLUKA CoA (Certificate of Analysis) is a formal document that attests to 00583_FLUKA's compliance with 00583_FLUKA specifications and serves as a tool for batch-level quality control.
00583_FLUKA CoA mostly includes findings from lab analyses of a specific batch. For each 00583_FLUKA CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
00583_FLUKA may be tested according to a variety of international standards, such as European Pharmacopoeia (00583_FLUKA EP), 00583_FLUKA JP (Japanese Pharmacopeia) and the US Pharmacopoeia (00583_FLUKA USP).

