
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
FDA Orange Book

0
Canada

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
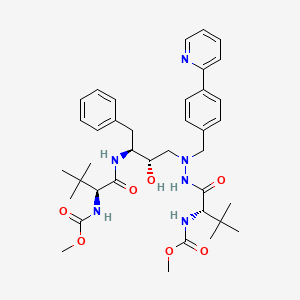

1. 232632, Bms
2. 3,12-bis(1,1-dimethylethyl)-8-hydroxy-4,11-dioxo-9-(phenylmethyl)-6-((4-(2-pyridinyl)phenyl)methyl)-2,5,6,10,13-pentaazatetradecanedioic Acid Dimethyl Ester
3. Atazanavir Sulfate
4. Bms 232632
5. Bms 232632 05
6. Bms-232632
7. Bms-232632-05
8. Bms232632
9. Bms23263205
10. Cgp 73547
11. Cgp 75136
12. Cgp 75176
13. Cgp 75355
14. Cgp-73547
15. Cgp-75136
16. Cgp-75176
17. Cgp-75355
18. Cgp73547
19. Cgp75136
20. Cgp75176
21. Cgp75355
22. Reyataz
1. 198904-31-3
2. Latazanavir
3. Zrivada
4. Reyataz
5. Bms-232632
6. Cgp 73547
7. Bms 232632
8. Cgp-73547
9. Atazanavir (inn)
10. Atv
11. Reyataz (tn)
12. Chembl1163
13. Qzu4h47a3s
14. Methyl N-[(2s)-1-[2-[(2s,3s)-2-hydroxy-3-[[(2s)-2-(methoxycarbonylamino)-3,3-dimethylbutanoyl]amino]-4-phenylbutyl]-2-[(4-pyridin-2-ylphenyl)methyl]hydrazinyl]-3,3-dimethyl-1-oxobutan-2-yl]carbamate
15. Chebi:37924
16. Ncgc00182552-01
17. Atazanavir [inn]
18. Atazanavir [inn:ban]
19. Dsstox_cid_28617
20. Dsstox_rid_82887
21. Dsstox_gsid_48691
22. (3s,8s,9s,12s)-3,12-bis(1,1-dimethylethyl)-8-hydroxy-4,11-dioxo-9-(phenylmethyl)-6-[[4-(2-pyridinyl)phenyl]methyl]-2,5,6,10,13-pentaazatetradecanedioic Acid Dimethyl Ester
23. Dr7
24. Methyl N-[1-[2-[2-hydroxy-3-[[2-(methoxycarbonylamino)-3,3-dimethylbutanoyl]amino]-4-phenylbutyl]-2-[(4-pyridin-2-ylphenyl)methyl]hydrazinyl]-3,3-dimethyl-1-oxobutan-2-yl]carbamate
25. Atazanavirum
26. Atazanavir Base
27. 1,14-dimethyl (3s,8s,9s,12s)-3,12-bis(1,1-dimethylethyl)-8-hydroxy-4,11-dioxo-9-(phenylmethyl)-6-[[4-(2-pyridinyl)phenyl]methyl]-2,5,6,10,13-pentaazatetradecanedioate
28. Methyl N-[(1s)-1-[[(1s,2s)-1-benzyl-2-hydroxy-3-[[[(2s)-2-(methoxycarbonylamino)-3,3-dimethyl-butanoyl]amino]-[[4-(2-pyridyl)phenyl]methyl]amino]propyl]carbamoyl]-2,2-dimethyl-propyl]carbamate
29. Methyl N-[(2s)-1-[[(2s,3s)-3-hydroxy-4-[[[(2s)-2-(methoxycarbonylamino)-3,3-dimethylbutanoyl]amino]-[(4-pyridin-2-ylphenyl)methyl]amino]-1-phenylbutan-2-yl]amino]-3,3-dimethyl-1-oxobutan-2-yl]carbamate
30. Cas-198904-31-3
31. Hsdb 7339
32. Unii-qzu4h47a3s
33. 2aqu
34. Reyataz(tm) (*1:1 Sulfate*)
35. (3s,8s,9s,12s)-3,12-bis(1,1-dimethylethyl)-8-hydroxy-4,11-dioxo-9-(phenylmethyl)-6-[[4-(2-pyridinyl)phenyl]methyl]-2,5, 6,10,13-pentaazatetradecanedioic Acid Dimethyl Ester
36. Atazanavir [mi]
37. Atazanavir [hsdb]
38. Atazanavir [vandf]
39. Atv & Aag
40. Atv & Hsa
41. Atazanavir [who-dd]
42. Schembl41696
43. Atazanavir,bisulfatesalt
44. Atazanavir, >=98% (hplc)
45. Dtxsid9048691
46. Atazanavir & Human Serum Albumin
47. Bdbm13934
48. Gtpl11138
49. Hms2089p22
50. Act06755
51. Amy31160
52. Zinc3941496
53. Tox21_113081
54. Mfcd08435966
55. Atazanavir & Alpha1-acid Glycoprotein
56. Akos025396423
57. Tox21_113081_1
58. Ccg-270390
59. Cs-0945
60. Db01072
61. Ncgc00182552-02
62. Ncgc00182552-03
63. Ncgc00182552-14
64. (2s)-n-(3-{[(2s)-2-(methoxycarbonylamino)-3,3-dimethylbutanoylamino][(4-(2-pyridyl)phenyl)methyl]amino}(1s,2s)-2-hydroxy-1-benzylpropyl)-2-(methoxycarbonylamino)-3,3-dimethylbutanamide
65. 2,5,6,10,13-pentaazatetradecanedioic Acid, 3,12-bis(1,1-dimethylethyl)-8-hydroxy-4,11-dioxo-9-(phenylmethyl)-6-((4-(2-pyridinyl)phenyl)methyl)-, Dimethyl Ester, (3s-(3r*,8r*,9r*,12r*))-
66. Bs-16315
67. Dimethyl (3s,8s,9s,12s)-9-benzyl-3,12-di-tert-butyl-8-hydroxy-4,11-dioxo-6-[4-(2-pyridyl)benzyl]-2,5,6,10,13-pentaazatetradecanedioate
68. Hy-17367
69. Methyl N-[(1s)-1-{n'-[(2s,3s)-2-hydroxy-3-[(2s)-2-[(methoxycarbonyl)amino]-3,3-dimethylbutanamido]-4-phenylbutyl]-n'-{[4-(pyridin-2-yl)phenyl]methyl}hydrazinecarbonyl}-2,2-dimethylpropyl]carbamate
70. S4662
71. A25022
72. D07471
73. Ab01274792-01
74. Ab01274792-02
75. Ab01274792-03
76. Ab01274792_04
77. Q423467
78. Sr-01000930924
79. J-519602
80. Sr-01000930924-2
81. 6,10,13-pentaazatetradecanedioic Acid Dimethyl Ester
82. (3s,8s,9s,12s)-3,12-bis(1,1-dimethylethyl)-8-hydroxy-4,11-dioxo-9-(phenylmethyl)-6-[[4-(2-pyridinyl)phenyl]methyl]-2,5,
83. 2,5,6,10,13-pentaazatetradecanedioic Acid, 3-12-bis(1,1-dimethylethyl)-8-hydroxy-4,11-dioxo-9-(phenylmethyl)-6-((-4-(2-pyridinyl)phenyl)methyl)-, Dimethyl Ester, (3s,8s,9s,12s)-
84. Atazanavir;(2s)-n-(3-{[(2s)-2-(methoxycarbonylamino)-3,3-dimethylbutanoylamino][(4-(2-pyridyl)phenyl)methyl]amino}(1s,2s)-2-hydroxy-1-benzylpropyl)-2-(methoxycarbonylamino)-3,3-dimethylbutanamide
85. Dimethyl (3s,8s,9s,12s)-9-benzyl-3,12,di-tert-butyl-8-hydroxy-4,11-dioxo-6-(p-2-pyridylbenzyl)-2,5,6,10,13-pentaazatetradecanedioate
86. Methyl [(1s,4s,5s,10s)-4-benzyl-1,10-di-tert-butyl-5-hydroxy-2,9,12-trioxo-7-(4-pyridin-2-ylbenzyl)-13-oxa-3,7,8,11-tetraazatetradec-1-yl]carbamate (non-preferred Name)
87. Methyl N-[(1s)-1-[[(2s,3s)-3-hydroxy-4-[[[(2s)-2-(methoxycarbonylamino)-3,3-dimethyl-butanoyl]amino]-[(4-pyridin-2-ylphenyl)methyl]amino]-1-phenyl-butan-2-yl]carbamoyl]-2,2-dimethyl-propyl]carbamate
88. Methyl N-[(1s)-1-{[(2s,3s)-3-hydroxy-4-[(2s)-2-[(methoxycarbonyl)amino]-3,3-dimethyl-n''-{[4-(pyridin-2-yl)phenyl]methyl}butanehydrazido]-1-phenylbutan-2-yl]carbamoyl}-2,2-dimethylpropyl]carbamate
89. Methyl N-[(2s)-1-[[(2s,3s)-3-hydroxy-4-[[[(2s)-2-(methoxycarbonylamino)-3,3-dimethylbutanoyl]amino]-[(4-pyridin-2-ylphenyl)methyl]amino]-1-phenylbutan-2-yl]ami
| Molecular Weight | 704.9 g/mol |
|---|---|
| Molecular Formula | C38H52N6O7 |
| XLogP3 | 5.6 |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 18 |
| Exact Mass | 704.38974802 g/mol |
| Monoisotopic Mass | 704.38974802 g/mol |
| Topological Polar Surface Area | 171 Ų |
| Heavy Atom Count | 51 |
| Formal Charge | 0 |
| Complexity | 1110 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Reyataz |
| PubMed Health | Atazanavir (By mouth) |
| Drug Classes | Antiretroviral Agent, Antiviral |
| Drug Label | REYATAZ (atazanavir sulfate) is an azapeptide inhibitor of HIV-1 protease.The chemical name for atazanavir sulfate is (3S,8S,9S,12S)-3,12-Bis(1,1-dimethylethyl)-8-hydroxy-4,11-dioxo-9-(phenylmethyl)-6-[[4-(2-pyridinyl)phenyl]methyl]-2,5,6,10,13-pen... |
| Active Ingredient | Atazanavir sulfate |
| Dosage Form | Capsule; Powder |
| Route | Oral |
| Strength | eq 50mg base/packet; eq 150mg base; eq 200mg base; eq 300mg base |
| Market Status | Prescription |
| Company | Bristol Myers Squibb |
| 2 of 2 | |
|---|---|
| Drug Name | Reyataz |
| PubMed Health | Atazanavir (By mouth) |
| Drug Classes | Antiretroviral Agent, Antiviral |
| Drug Label | REYATAZ (atazanavir sulfate) is an azapeptide inhibitor of HIV-1 protease.The chemical name for atazanavir sulfate is (3S,8S,9S,12S)-3,12-Bis(1,1-dimethylethyl)-8-hydroxy-4,11-dioxo-9-(phenylmethyl)-6-[[4-(2-pyridinyl)phenyl]methyl]-2,5,6,10,13-pen... |
| Active Ingredient | Atazanavir sulfate |
| Dosage Form | Capsule; Powder |
| Route | Oral |
| Strength | eq 50mg base/packet; eq 150mg base; eq 200mg base; eq 300mg base |
| Market Status | Prescription |
| Company | Bristol Myers Squibb |
Atazanavir sulfate is indicated in combination with other antiretroviral agents for the treatment of HIV-1 infection. The use of atazanavir sulfate may be considered in antiretroviral-treatment experienced adults with HIV strains that are expected to be susceptible to atazanavir sulfate by genotypic and phenotypic testing. /Included in US product labeling/
Thomson.Micromedex. Drug Information for the Health Care Professional. 25th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2005., p. 457
Lactic acidosis syndrome, sometimes fatal, and symptomatic hyperlactatemia have been reported in patients receiving atazanavir in conjunction with nucleoside reverse transcriptase inhibitors (NRTIs). Therapy with NRTIs is known to be associated with an increased risk of lactic acidosis syndrome; female gender and obesity also are known risk factors for this syndrome. Whether atazanavir contributes to the risk of lactic acidosis syndrome remains to be established.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 629
Hyperglycemia (potentially persistent), new-onset diabetes mellitus, or exacerbation of preexisting diabetes mellitus has been reported in patients receiving HIV protease inhibitors. May require initiation of antidiabetic therapy (e.g., insulin, oral antidiabetic agents) or dosage adjustment for existing diabetes; diabetic ketoacidosis can occur.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 629
Abnormalities in AV conduction, including prolongation of the PR interval, have occurred in individuals receiving atazanavir. Cardiac conduction abnormalities generally are limited to first-degree AV block; prolongation of the QTc interval observed in HIV-infected patients receiving atazanavir have not been directly attributed to the drug. Asymptomatic first-degree AV block was observed in 5.9 or 3-10.4% of patients in clinical trials receiving regimens that included atazanavir or comparator antiretrovirals (lopinavir/ritonavir, nelfinavir, efavirenz), respectively; second- or third-degree block was not observed. Atazanavir should be used with caution in patients with cardiac conduction abnormalities (e.g., marked first-degree AV block; second- or third-degree AV block) because of lack of clinical experience.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 629
Because atazanavir is a competitive inhibitor of uridine diphosphate-glucuronosyltransferase (UGT) 1A1 (an enzyme that catalyzes the glucuronidation of bilirubin), reversible asymptomatic elevations in indirect (unconjugated) bilirubin occur in most patients receiving the drug. Total bilirubin concentrations at least 2.6 times the upper limit of normal have been reported in 35-47% of patients receiving the drug in clinical trials; long-term safety data are not available for patients experiencing persistent elevations in total bilirubin exceeding 5 times the upper limit of normal. Increases in serum AST (SGOT) and/or ALT (SGPT) concentrations that occur with hyperbilirubinemia should be evaluated for etiologies other than hyperbilirubinemia. If jaundice or scleral icterus that result from bilirubin elevations cause cosmetic concerns, alternative antiretroviral therapy can be considered; reduction of atazanavir dosage not recommended (efficacy data not available for reduced dosages).
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 629
For more Drug Warnings (Complete) data for ATAZANAVIR (17 total), please visit the HSDB record page.
Atazanavir is indicated in combination with other antiretroviral agents for the treatment of HIV-1 infection in adults and pediatric patients 3 months of age and older weighing at least 5kg. Atazanavir is also indicated in combination with [cobicistat] and other antiretrovirals for the treatment of HIV-1 infection in adults and pediatric patients weighing at least 35kg.
FDA Label
Atazanavir Mylan, co-administered with low dose ritonavir, is indicated for the treatment of HIV 1 infected adults and paediatric patients 6 years of age and older in combination with other antiretroviral medicinal products.
Based on available virological and clinical data from adult patients, no benefit is expected in patients with strains resistant to multiple protease inhibitors ( 4 PI mutations). There are very limited data available from children aged 6 to less than 18 years.
The choice of Atazanavir Mylan in treatment experienced adult and paediatric patients should be based on individual viral resistance testing and the patients treatment history.
Reyataz capsules, co-administered with low dose ritonavir, are indicated for the treatment of HIV-1 infected adults and paediatric patients 6 years of age and older in combination with other antiretroviral medicinal products (see section 4. 2).
Based on available virological and clinical data from adult patients, no benefit is expected in patients with strains resistant to multiple protease inhibitors ( 4 PI mutations).
The choice of Reyataz in treatment experienced adult and paediatric patients should be based on individual viral resistance testing and the patients treatment history (see sections 4. 4 and 5. 1).
Reyataz oral powder, co-administered with low dose ritonavir, is indicated in combination with other antiretroviral medicinal products for the treatment of HIV-1 infected paediatric patients at least 3 months of age and weighing at least 5 kg (see section 4. 2).
Based on available virological and clinical data from adult patients, no benefit is expected in patients with strains resistant to multiple protease inhibitors ( 4 PI mutations). The choice of Reyataz in treatment experienced adult and paediatric patients should be based on individual viral resistance testing and the patients treatment history (see sections 4. 4 and 5. 1).
Atazanavir Krka capsules, co-administered with low dose ritonavir, are indicated for the treatment of HIV-1 infected adults and paediatric patients 6 years of age and older in combination with other antiretroviral medicinal products.
Based on available virological and clinical data from adult patients, no benefit is expected in patients with strains resistant to multiple protease inhibitors ( 4 PI mutations).
The choice of Atazanavir Krka in treatment experienced adult and paediatric patients should be based on individual viral resistance testing and the patients treatment history.
Atazanavir (ATV) is an azapeptide HIV-1 protease inhibitor (PI) with activity against Human Immunodeficiency Virus Type 1 (HIV-1). HIV-1 protease is an enzyme required for the proteolytic cleavage of the viral polyprotein precursors into the individual functional proteins found in infectious HIV-1. Atazanavir binds to the protease active site and inhibits the activity of the enzyme. This inhibition prevents cleavage of the viral polyproteins resulting in the formation of immature non-infectious viral particles. Protease inhibitors are almost always used in combination with at least two other anti-HIV drugs. Atazanivir is pharmacologically related but structurally different from other protease inhibitors and other currently available antiretrovirals.
HIV Protease Inhibitors
Inhibitors of HIV PROTEASE, an enzyme required for production of proteins needed for viral assembly. (See all compounds classified as HIV Protease Inhibitors.)
J05AE08
J05AE08
J05AE08
J - Antiinfectives for systemic use
J05 - Antivirals for systemic use
J05A - Direct acting antivirals
J05AE - Protease inhibitors
J05AE08 - Atazanavir
Absorption
Atazanavir is rapidly absorbed with a Tmax of approximately 2.5 hours. Administration of atazanavir with food enhances bioavailability and reduces pharmacokinetic variability. Oral bioavailability is 60-68%.
Atazanavir is rapidly absorbed with a Tmax of approximately 2.5 hours. Atazanavir demonstrates nonlinear pharmacokinetics with greater than dose-proportional increases in AUC and Cmax values over the dose range of 200-800 mg once daily. Steady-state is achieved between Days 4 and 8, with an accumulation of approximately 2.3-fold.
Physicians Desk Reference. 59th ed. Thomson PDR. Montvale, NJ 2005., p. 1061
Administration of /atazanavir/ with food enhances bioavailability and reduces pharmacokinetic variability. Administration of a single dose of /atazanavir/ with a light meal (357 kcal, 8.2 g fat, 10.6 g protein) resulted in a 70% increase in AUC and 57% increase in Cmax relative to the fasting state. Administration of a single dose of /atazanavir/ with a high-fat meal (721 kcal, 37.3 g fat, 29.4 g protein) resulted in a mean increase in AUC of 35% with no change in Cmax relative to the fasting state. Administration of /atazanavir/ with either a light meal or high-fat meal decreased the coefficient of variation of AUC and Cmax by approximately one half compared to the fasting state.
Physicians Desk Reference. 59th ed. Thomson PDR. Montvale, NJ 2005., p. 1061
Peak plasma concentration: Healthy subjects: 5199 ng/mL on day 29 following a 400 mg daily dose with a light meal. HIV-infected patients: 2298 ng/mL on day 29 following a 400 mg daily dose with a light meal.
Thomson.Micromedex. Drug Information for the Health Care Professional. 25th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2005., p. 457
Time to peak concentration: HIV-infected patients: 2 hours.
Thomson.Micromedex. Drug Information for the Health Care Professional. 25th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2005., p. 457
For more Absorption, Distribution and Excretion (Complete) data for ATAZANAVIR (8 total), please visit the HSDB record page.
Atazanavir is extensively metabolized in humans, primarily by the liver. The major biotransformation pathways of atazanavir in humans consisted of monooxygenation and dioxygenation. Other minor biotransformation pathways for atazanavir or its metabolites consisted of glucuronidation, N-dealkylation, hydrolysis, and oxygenation with dehydrogenation. In vitro studies using human liver microsomes suggested that atazanavir is metabolized by CYP3A.
Atazanavir is extensively metabolized in humans. The major biotransformation pathways of atazanavir in humans consisted of monooxygenation and (atazanavir sulfate) dioxygenation. Other minor biotransformation pathways for atazanavir or its metabolites consisted of glucuronidation, N-dealkylation, hydrolysis, and oxygenation with dehydrogenation. Two minor metabolites of atazanavir in plasma have been characterized. Neither metabolite demonstrated in vitro antiviral activity. In vitro studies using human liver microsomes suggested that atazanavir is metabolized by CYP3A.
Physicians Desk Reference. 59th ed. Thomson PDR. Montvale, NJ 2005., p. 1062
Elimination half-life in adults (healthy and HIV infected) is approximately 7 hours (following a 400 mg daily dose with a light meal). Elimination half-life in hepatically impaired is 12.1 hours (following a single 400 mg dose).
The mean half-life of atazanavir in hepatically impaired subjects was 12.1 hours compared with 6.4 hours in healthy volunteers. ...
Physicians Desk Reference. 59th ed. Thomson PDR. Montvale, NJ 2005., p. 1062
The mean elimination half-life of atazanavir in healthy volunteers (n=214) and HIV-infected adult patients (n=13) was approximately 7 hours at steady state following a dose of 400 mg daily with a light meal.
Physicians Desk Reference. 59th ed. Thomson PDR. Montvale, NJ 2005., p. 1062
Atazanavir selectively inhibits the virus-specific processing of viral Gag and Gag-Pol polyproteins in HIV-1 infected cells by binding to the active site of HIV-1 protease, thus preventing the formation of mature virions. Atazanavir is not active against HIV-2.
Atazanavir is an azapeptide HIV-1 protease inhibitor. The compound selectively inhibits the virus-specific processing of viral Gag and Gag-Pol polyproteins in HIV-1 infected cells, thus preventing formation of mature virions.
Physicians Desk Reference. 59th ed. Thomson PDR. Montvale, NJ 2005., p. 1060
BMS-232632 is an azapeptide human immunodeficiency virus type 1 (HIV-1) protease (Prt) inhibitor that exhibits potent anti-HIV activity with a 50% effective concentration (EC(50)) of 2.6 to 5.3 nM and an EC(90) of 9 to 15 nM in cell culture. Proof-of-principle studies indicate that BMS-232632 blocks the cleavage of viral precursor proteins in HIV-infected cells, proving that it functions as an HIV Prt inhibitor. Comparative studies showed that BMS-232632 is generally more potent than the five currently approved HIV-1 Prt inhibitors. Furthermore, BMS-232632 is highly selective for HIV-1 Prt and exhibits cytotoxicity only at concentrations 6,500- to 23, 000-fold higher than that required for anti-HIV activity. To assess the potential of this inhibitor when used in combination with other antiretrovirals, BMS-232632 was evaluated for anti-HIV activity in two-drug combination studies. Combinations of BMS-232632 with either stavudine, didanosine, lamivudine, zidovudine, nelfinavir, indinavir, ritonavir, saquinavir, or amprenavir in HIV-infected peripheral blood mononuclear cells yielded additive to moderately synergistic antiviral effects. Importantly, combinations of drug pairs did not result in antagonistic anti-HIV activity or enhanced cytotoxic effects at the highest concentrations used for antiviral evaluation. Our results suggest that BMS-232632 may be an effective HIV-1 inhibitor that may be utilized in a variety of different drug combinations.
PMID:10898681 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC90019 Robinson BS et al; Antimicrob Agents Chemother 44 (8): 2093-9 (2000)

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
63
PharmaCompass offers a list of Atazanavir API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Atazanavir manufacturer or Atazanavir supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Atazanavir manufacturer or Atazanavir supplier.
PharmaCompass also assists you with knowing the Atazanavir API Price utilized in the formulation of products. Atazanavir API Price is not always fixed or binding as the Atazanavir Price is obtained through a variety of data sources. The Atazanavir Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Atazanavir manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Atazanavir, including repackagers and relabelers. The FDA regulates Atazanavir manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Atazanavir API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Atazanavir manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Atazanavir supplier is an individual or a company that provides Atazanavir active pharmaceutical ingredient (API) or Atazanavir finished formulations upon request. The Atazanavir suppliers may include Atazanavir API manufacturers, exporters, distributors and traders.
click here to find a list of Atazanavir suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
Atazanavir Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Atazanavir GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Atazanavir GMP manufacturer or Atazanavir GMP API supplier for your needs.
A Atazanavir CoA (Certificate of Analysis) is a formal document that attests to Atazanavir's compliance with Atazanavir specifications and serves as a tool for batch-level quality control.
Atazanavir CoA mostly includes findings from lab analyses of a specific batch. For each Atazanavir CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Atazanavir may be tested according to a variety of international standards, such as European Pharmacopoeia (Atazanavir EP), Atazanavir JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Atazanavir USP).
