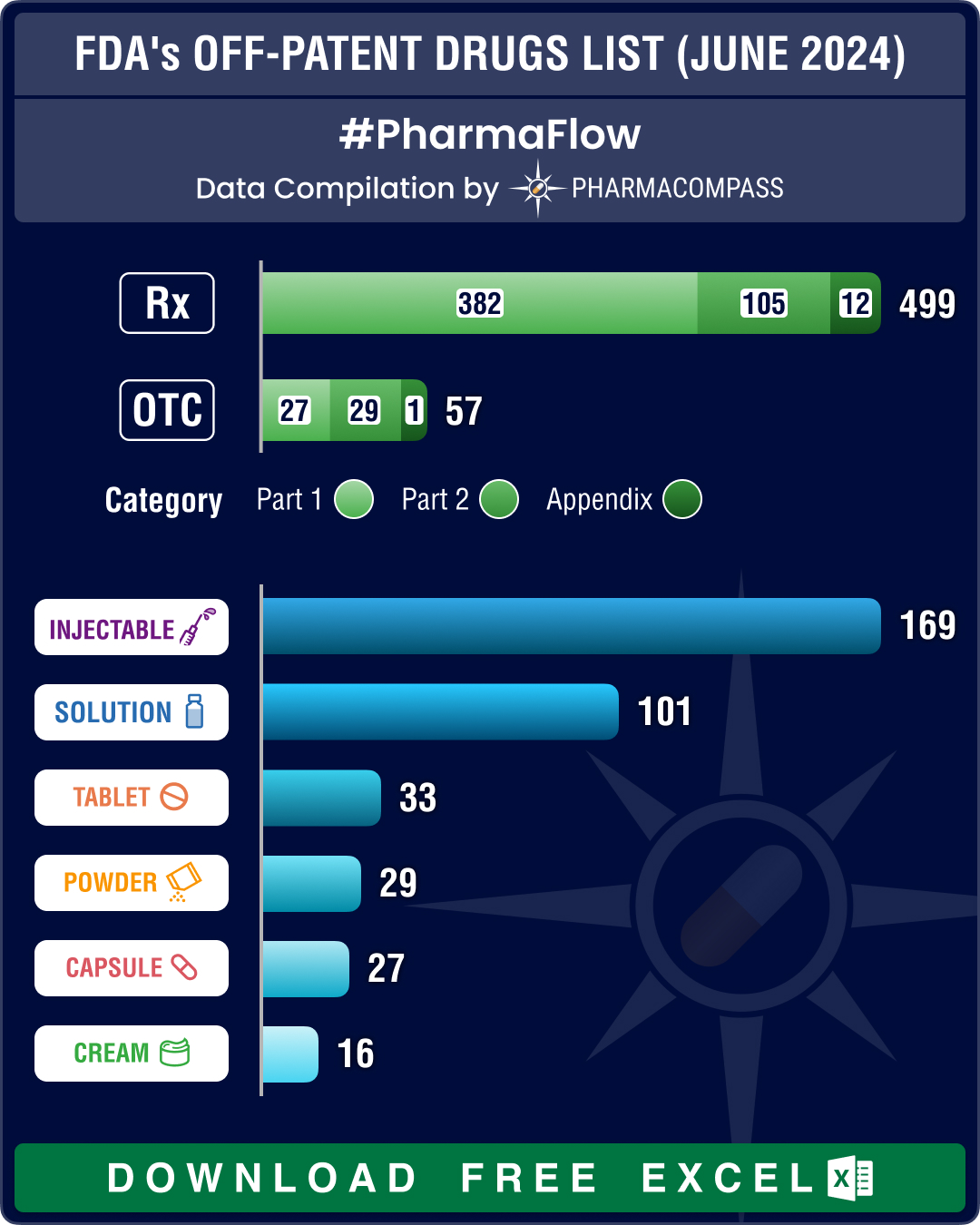
FDA’s December 2025 OPOE list features 784 prescription drugs, 73 OTC drugs
This
week, PharmaCompass brings you key highlights of the US Food and Drug Administration’s D
Excipient Market Overview: Roquette, Seqens, Evonik make strategic moves; new guidelines deal with contamination
The pharmaceutical industry has long recognized the critical role
excipients or inactive ingredient


 Market Place
Market Place Sourcing Support
Sourcing Support