NDC Code(s) : 69097-677-02, 69097-677-05, 69097-677-12, 69097-677-19, 69097-677-21, 69097-678-02, 69097-678-05, 69097-678-12, 69097-678-19, 69097-678-21, 69097-679-02, 69097-679-05, 69097-679-12, 69097-679-19, 69097-679-21, 69097-681-02, 69097-681-05, 69097-681-12, 69097-681-19, 69097-681-21, 69097-682-02, 69097-682-05, 69097-682-12, 69097-682-19, 69097-682-21, 69097-683-02, 69097-683-05, 69097-683-12, 69097-683-19, 69097-683-21, 69097-684-02, 69097-684-05, 69097-684-12, 69097-684-19, 69097-684-21, 69097-685-02, 69097-685-05, 69097-685-12, 69097-685-19, 69097-685-21
Packager : Cipla USA Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : CV
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| PREGABALIN capsules, CV PREGABALIN capsules, CV CAPSULE | |||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
| PREGABALIN capsules, CV PREGABALIN capsules, CV CAPSULE | |||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
| PREGABALIN capsules, CV PREGABALIN capsules, CV CAPSULE | |||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
| PREGABALIN capsules, CV PREGABALIN capsules, CV CAPSULE | |||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
| PREGABALIN capsules, CV PREGABALIN capsules, CV CAPSULE | |||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
| PREGABALIN capsules, CV PREGABALIN capsules, CV CAPSULE | |||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
| PREGABALIN capsules, CV PREGABALIN capsules, CV CAPSULE | |||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
| PREGABALIN capsules, CV PREGABALIN capsules, CV CAPSULE | |||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
| LABELER - Cipla USA Inc.(078719707) |
| REGISTRANT - Cipla USA Inc.(078719707) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Cipla Limited- Kurkumbh | 917066446 | MANUFACTURE(69097-677, 69097-678, 69097-679, 69097-681, 69097-682, 69097-683, 69097-684, 69097-685) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| MSN Pharmachem Private Limited | 677318200 | ANALYSIS(69097-677, 69097-678, 69097-679, 69097-681, 69097-682, 69097-683, 69097-684, 69097-685), API MANUFACTURE(69097-677, 69097-678, 69097-679, 69097-681, 69097-682, 69097-683, 69097-684, 69097-685) | |
PRINCIPAL DISPLAY PANEL
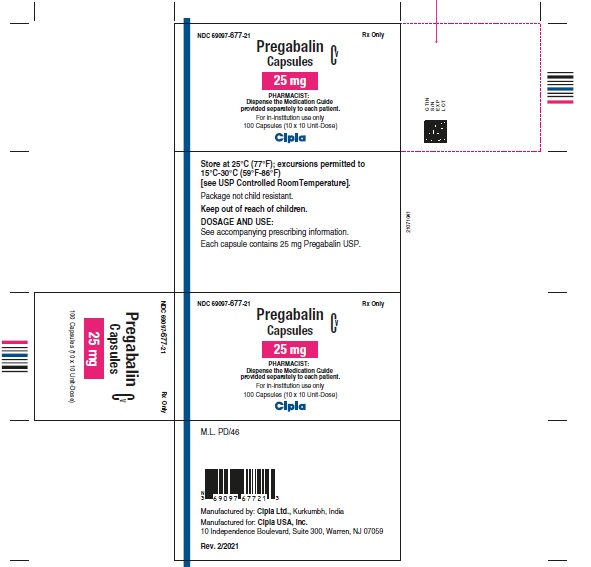
NDC 69097- 677-21 Rx Only
Pregabalin Cv
Capsules
25 mg
PHARMACIST :
Dispense the Medication Guide
Provided separately to each patient
For in-institution use only
100 Capsules (10 x 10 Unit-Dose)
Cipla
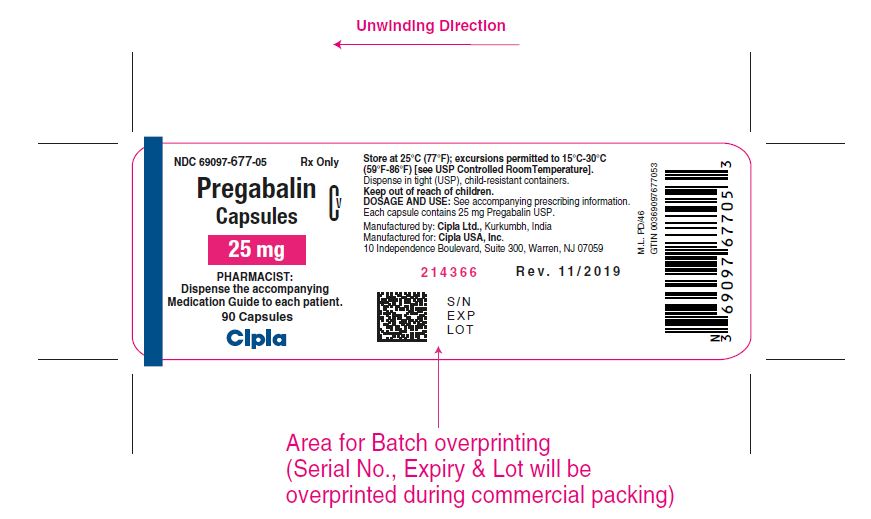
NDC 69097- 677-05 Rx Only
Pregabalin Cv
Capsules
25 mg
PHARMACIST :
Dispense the accompanying
Medication Guide to each patient
90 capsules
Cipla
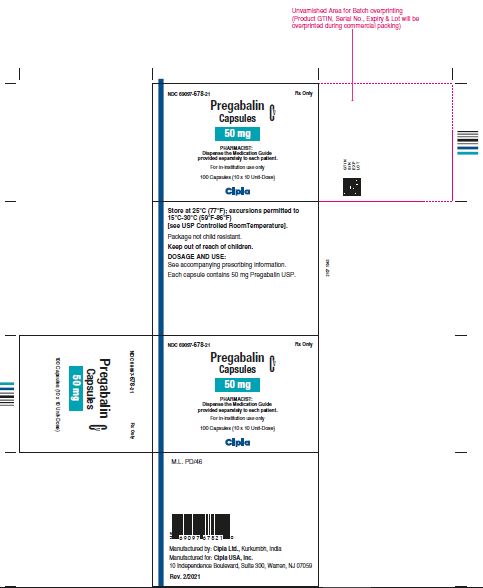
NDC 69097- 678-21 Rx Only
Pregabalin Cv
Capsules
50 mg
PHARMACIST :
Dispense the Medication Guide
Provided separately to each patient
For in-institution use only
100 Capsules (10 x 10 Unit-Dose)
Cipla
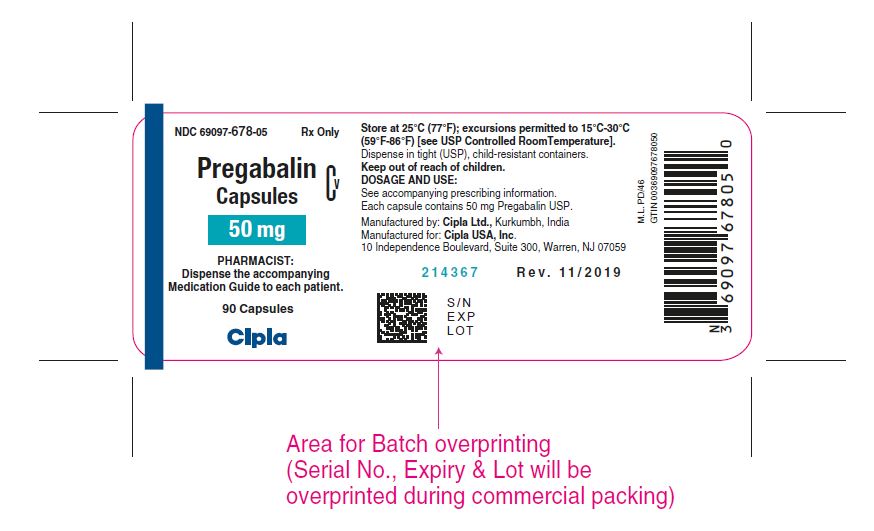
NDC 69097- 678-0 5 Rx Only
Pregabalin Cv
Capsules
50 mg
PHARMACIST :
Dispense the accompanying
Medication Guide to each patient
90 capsules
Cipla
NDC 69097- 679-21 Rx Only
Pregabalin Cv
Capsules
75 mg
PHARMACIST :
Dispense the Medication Guide
Provided separately to each patient
For in-institution use only
100 Capsules (10 x 10 Unit-Dose)
Cipla
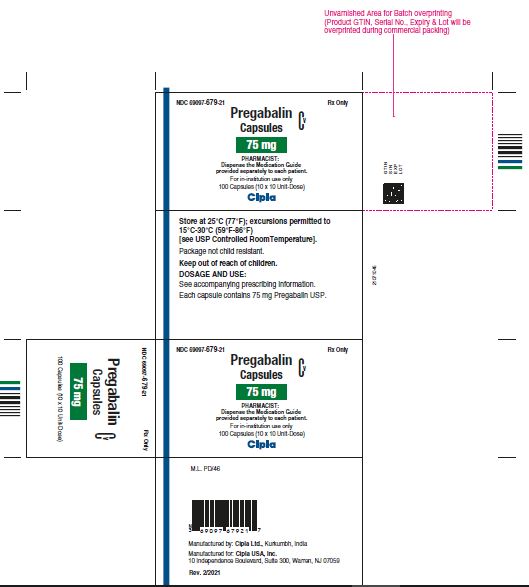
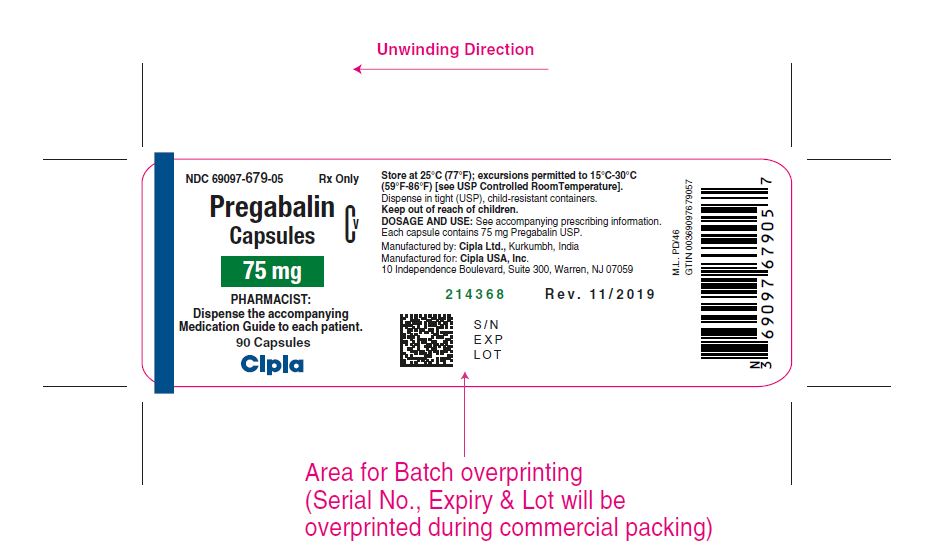
NDC 69097- 679-05 Rx Only
Pregabalin Cv
Capsules
75 mg
PHARMACIST :
Dispense the accompanying
Medication Guide to each patient
90 capsules
Cipla
NDC 69097- 681-21 Rx Only
Pregabalin Cv
Capsules
100 mg
PHARMACIST :
Dispense the Medication Guide
Provided separately to each patient
For in-institution use only
100 Capsules (10 x 10 Unit-Dose)
Cipla
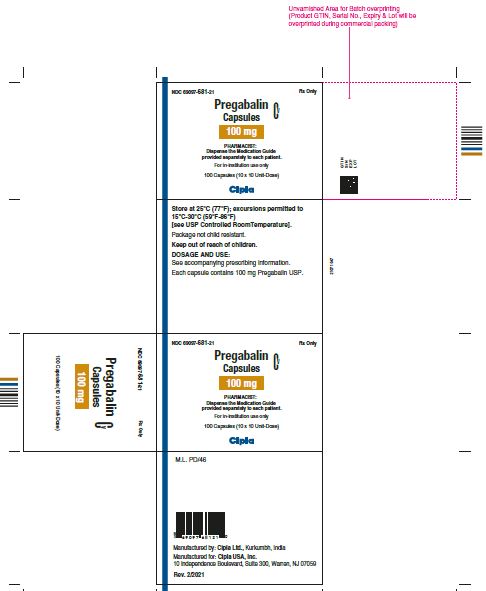
NDC 69097- 681-05 Rx Only
Pregabalin Cv
Capsules
100 mg
PHARMACIST :
Dispense the accompanying
Medication Guide to each patient
90 capsules
Cipla

NDC 69097- 682-21 Rx Only
Pregabalin Cv
Capsules
150 mg
PHARMACIST :
Dispense the Medication Guide
Provided separately to each patient
For in-institution use only
100 Capsules (10 x 10 Unit-Dose)
Cipla
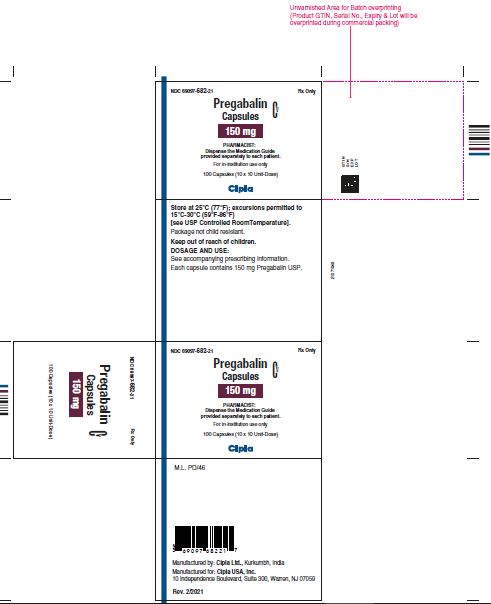
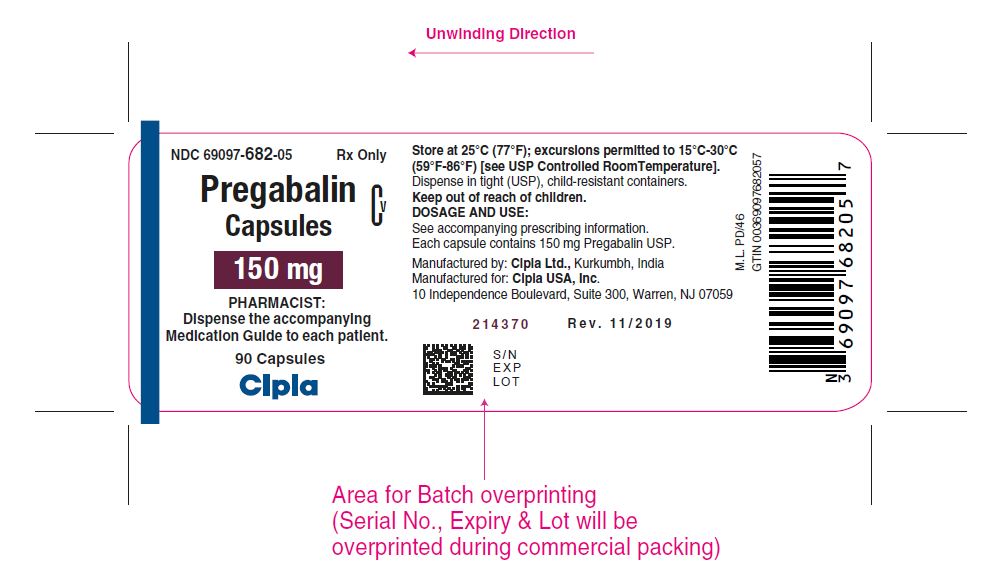
NDC 69097- 682-05 Rx Only
Pregabalin Cv
Capsules
150 mg
PHARMACIST :
Dispense the accompanying
Medication Guide to each patient
90 capsules
Cipla
NDC 69097- 683-21 Rx Only
Pregabalin Cv
Capsules
200 mg
PHARMACIST :
Dispense the Medication Guide
Provided separately to each patient
For in-institution use only
100 Capsules (10 x 10 Unit-Dose)
Cipla
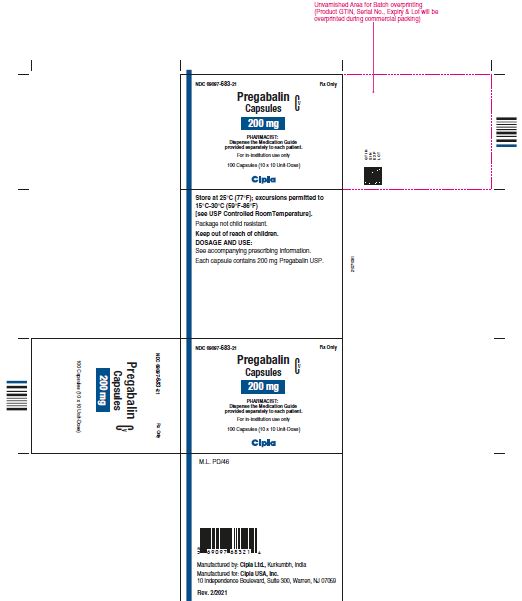
NDC 69097- 683-05 Rx Only
Pregabalin Cv
Capsules
200 mg
PHARMACIST :
Dispense the accompanying
Medication Guide to each patient
90 capsules
Cipla

NDC 69097- 684-21 Rx Only
Pregabalin Cv
Capsules
225 mg
PHARMACIST :
Dispense the Medication Guide
Provided separately to each patient
For in-institution use only
100 Capsules (10 x 10 Unit-Dose)
Cipla
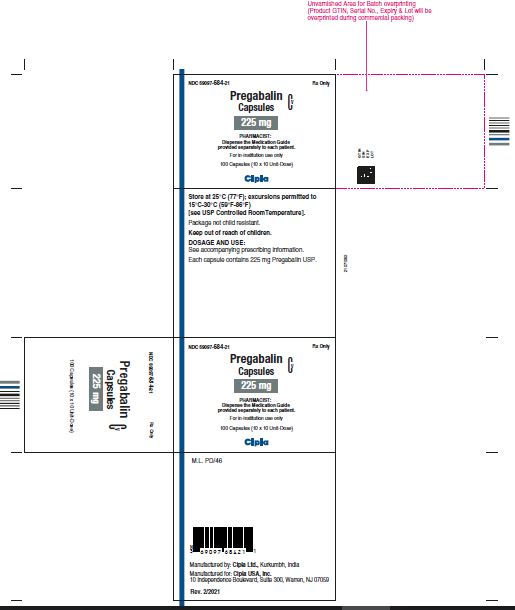
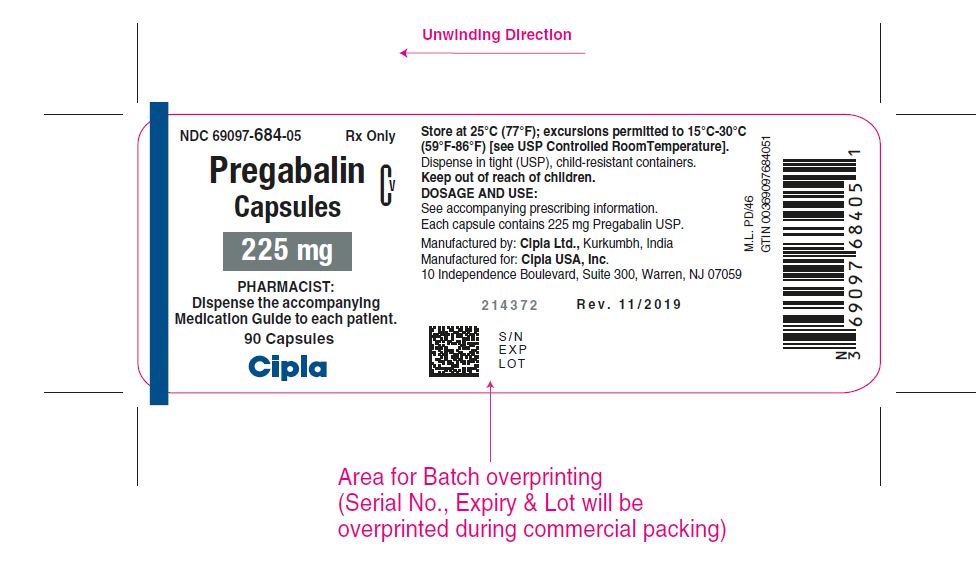
NDC 69097- 684-05 Rx Only
Pregabalin Cv
Capsules
225 mg
PHARMACIST :
Dispense the accompanying
Medication Guide to each patient
90 capsules
Cipla
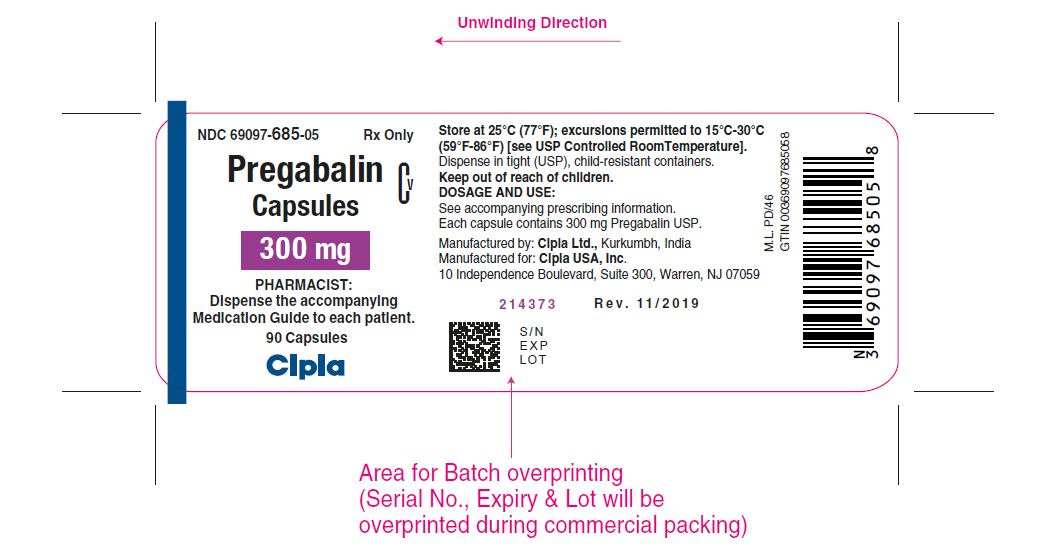
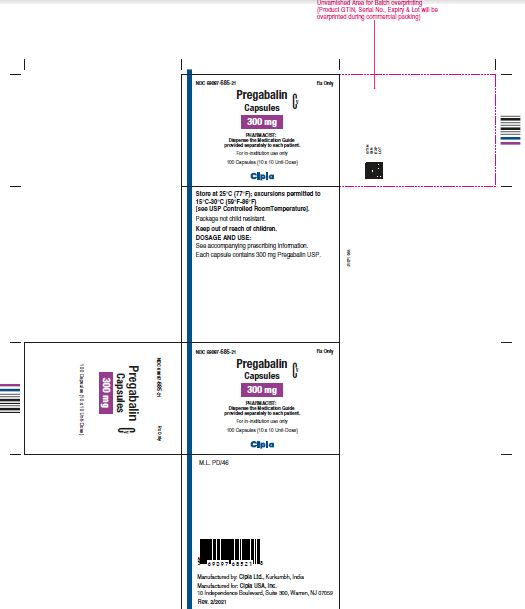
NDC 69097- 685-21 Rx Only
Pregabalin Cv
Capsules
300 mg
PHARMACIST :
Dispense the Medication Guide
Provided separately to each patient
For in-institution use only
100 Capsules (10 x 10 Unit-Dose)
Cipla
NDC 69097- 685-05 Rx Only
Pregabalin Cv
Capsules
300mg
PHARMACIST :
Dispense the accompanying
Medication Guide to each patient
90 capsules
Cipla