NDC Code(s) : 69097-524-44
Packager : Cipla USA Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Diclofenac Sodium Diclofenac Sodium GEL | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LABELER - Cipla USA Inc.(078719707) |
| REGISTRANT - Cipla USA Inc.(078719707) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Cipla Ltd. Goa | 650072015 | MANUFACTURE(69097-524) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| DPT Laboratories Ltd. | 832224526 | MANUFACTURE(69097-524), ANALYSIS(69097-524) | |
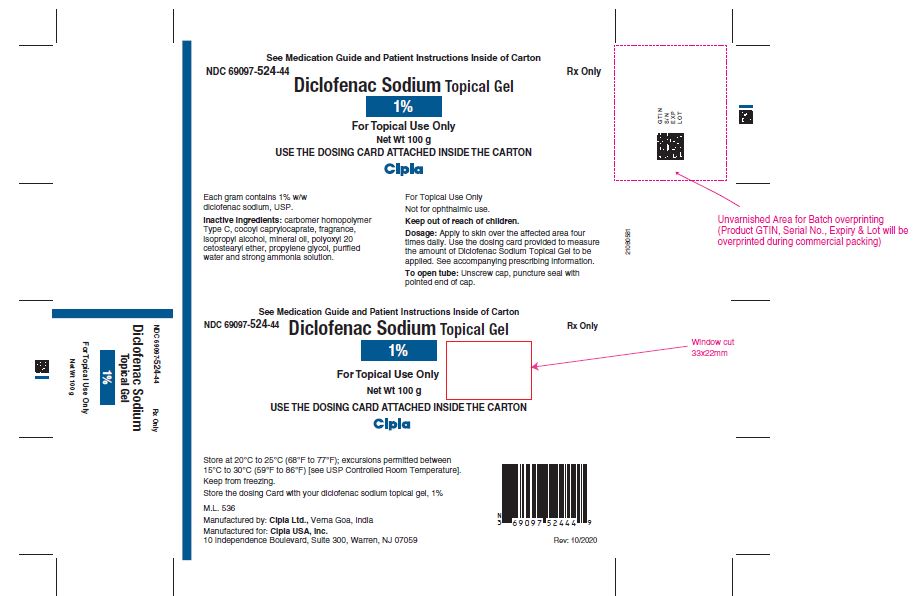
PRINCIPAL DISPLAY PANEL
NDC 69097-524-44
Diclofenac Sodium Topical Gel
1%
For Topical Use Only
Net Wt 100 g
USE THE DOSING CARD ATTACHED INSIDE THE CARTON
Cipla
 carton-label
carton-label









