NDC Code(s) : 68745-2080-1, 68745-2080-2, 68745-2081-1, 68745-2081-2, 68745-2082-1, 68745-2082-2, 68745-2083-1, 68745-2083-2, 68745-2084-1, 68745-2084-2, 68745-2085-1, 68745-2085-2, 68745-2086-1, 68745-2086-2, 68745-2087-1, 68745-2087-2, 68745-2088-1, 68745-2088-2, 68745-2089-1, 68745-2089-2
Packager : CHANEL PARFUMS BEAUTE
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Les Beiges Octinoxate and Titanium Dioxide GEL | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Les Beiges Octinoxate and Titanium dioxide GEL | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Les Beiges Octinoxate and Titanium dioxide GEL | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Les Beiges Octinoxate and Titanium dioxide GEL | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Les Beiges Octinoxate and Titanium dioxide GEL | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Les Beiges Octinoxate and Titanium dioxide GEL | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Les Beiges Octinoxate and Titanium dioxide GEL | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Les Beiges Octinoxate and Titanium dioxide GEL | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Les Beiges Octinoxate and Titanium dioxide GEL | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Les Beiges Octinoxate and Titanium dioxide GEL | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
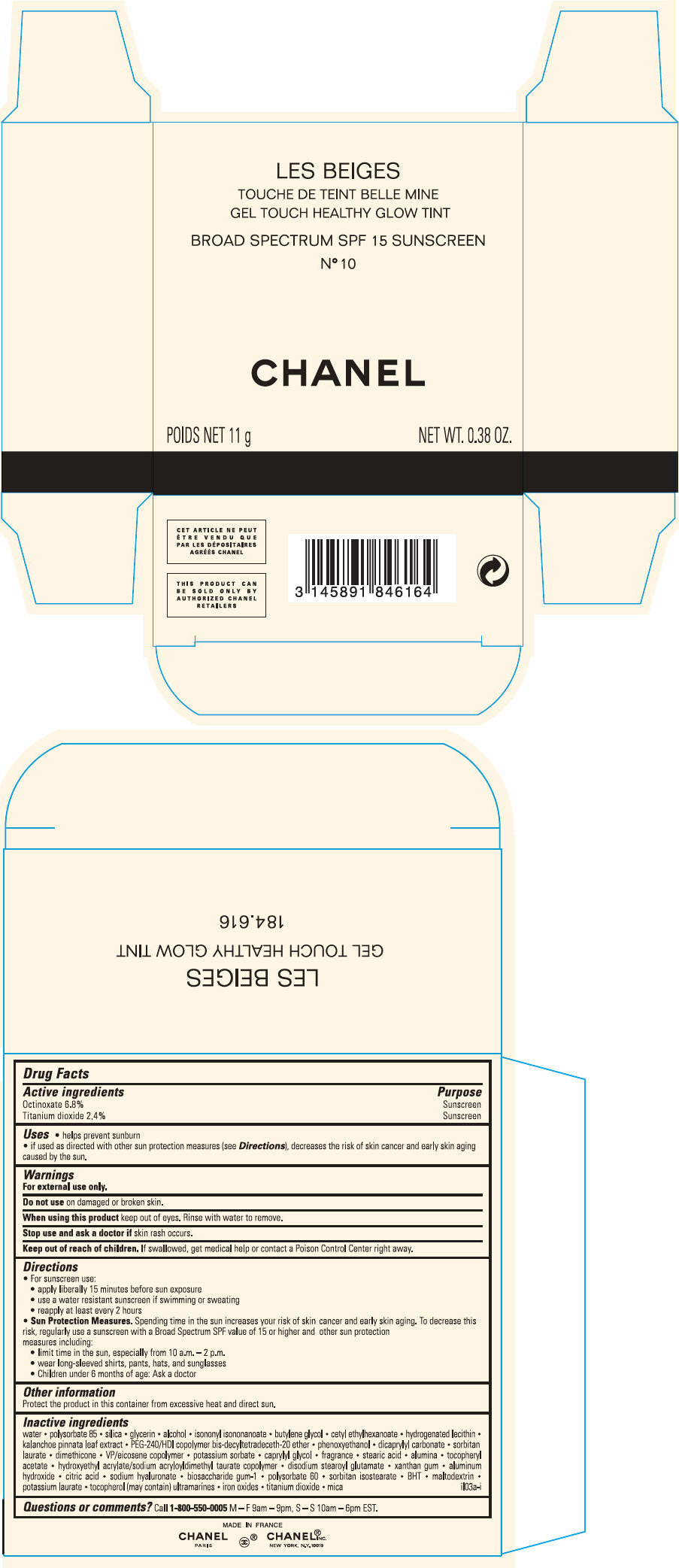
PRINCIPAL DISPLAY PANEL
LES BEIGES
GEL TOUCH HEALTHY GLOW TINT
BROAD SPECTRUM SPF 15 SUNSCREEN
N° 10
CHANEL
POIDS NET 11 g
NET WT. 0.38 OZ.
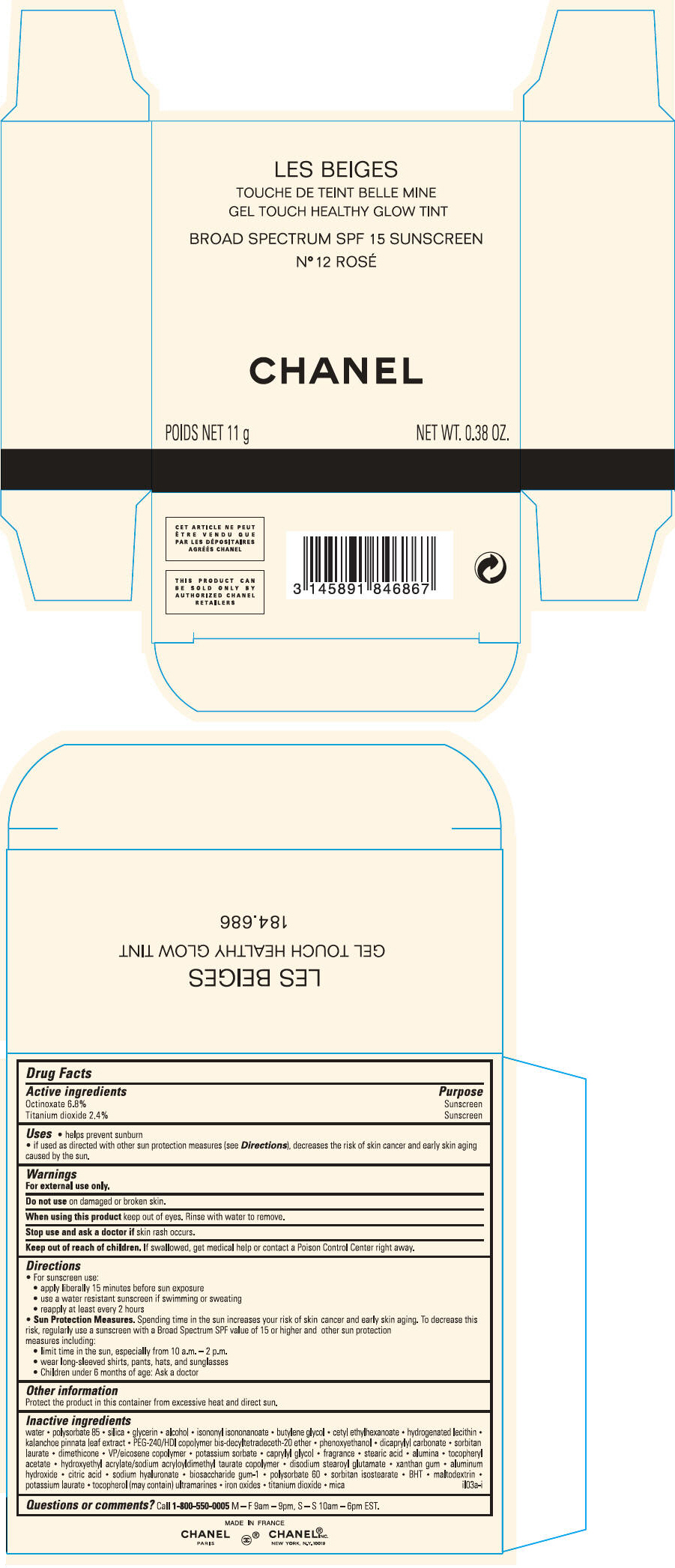
PRINCIPAL DISPLAY PANEL
LES BEIGES
GEL TOUCH HEALTHY GLOW TINT
BROAD SPECTRUM SPF 15 SUNSCREEN
N° 12 ROSÉ
CHANEL
POIDS NET 11 g
NET WT. 0.38 OZ.
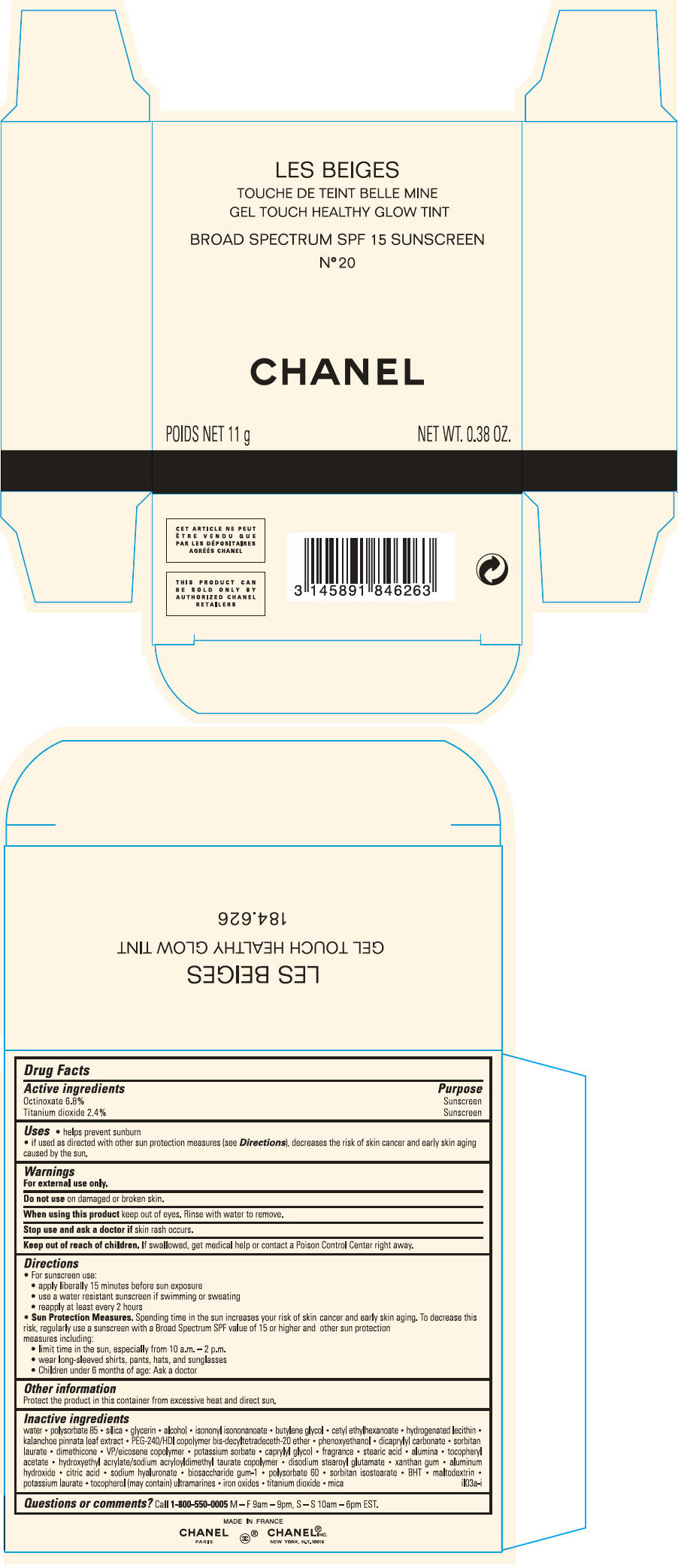
PRINCIPAL DISPLAY PANEL
LES BEIGES
GEL TOUCH HEALTHY GLOW TINT
BROAD SPECTRUM SPF 15 SUNSCREEN
N° 20
CHANEL
POIDS NET 11 g
NET WT. 0.38 OZ.
PRINCIPAL DISPLAY PANEL
LES BEIGES
GEL TOUCH HEALTHY GLOW TINT
BROAD SPECTRUM SPF 15 SUNSCREEN
N° 21
CHANEL
POIDS NET 11 g
NET WT. 0.38 OZ.
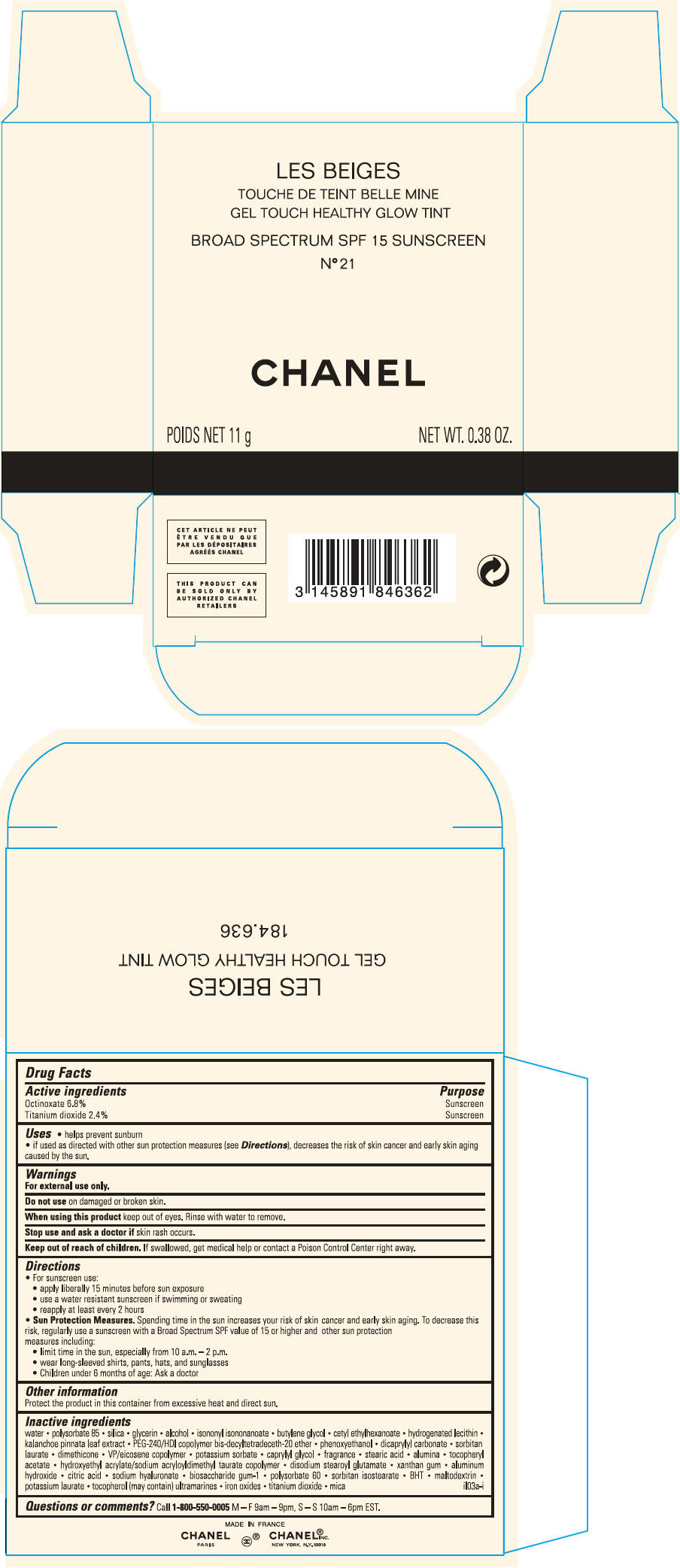
PRINCIPAL DISPLAY PANEL
LES BEIGES
GEL TOUCH HEALTHY GLOW TINT
BROAD SPECTRUM SPF 15 SUNSCREEN
N° 22 ROSÉ
CHANEL
POIDS NET 11 g
NET WT. 0.38 OZ.
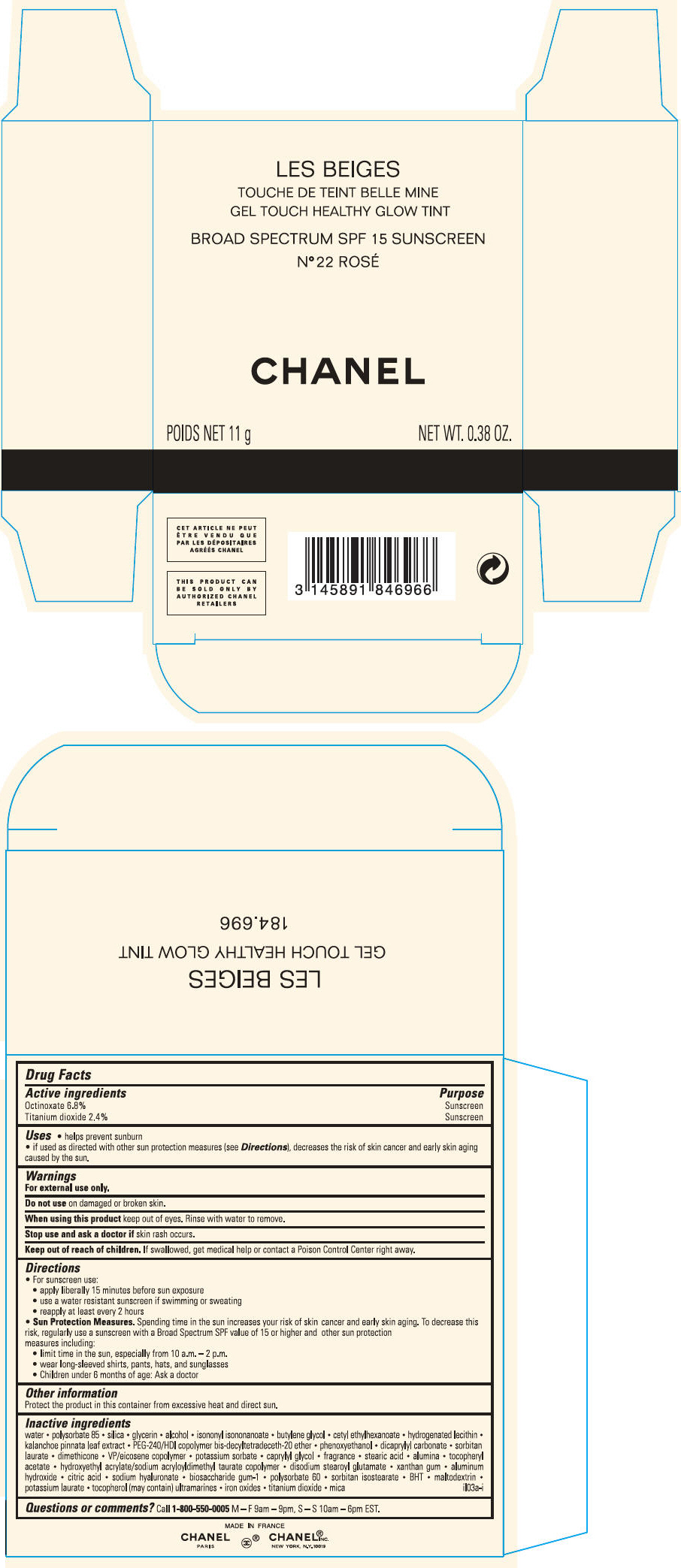
PRINCIPAL DISPLAY PANEL
LES BEIGES
GEL TOUCH HEALTHY GLOW TINT
BROAD SPECTRUM SPF 15 SUNSCREEN
N° 30
CHANEL
POIDS NET 11 g
NET WT. 0.38 OZ.
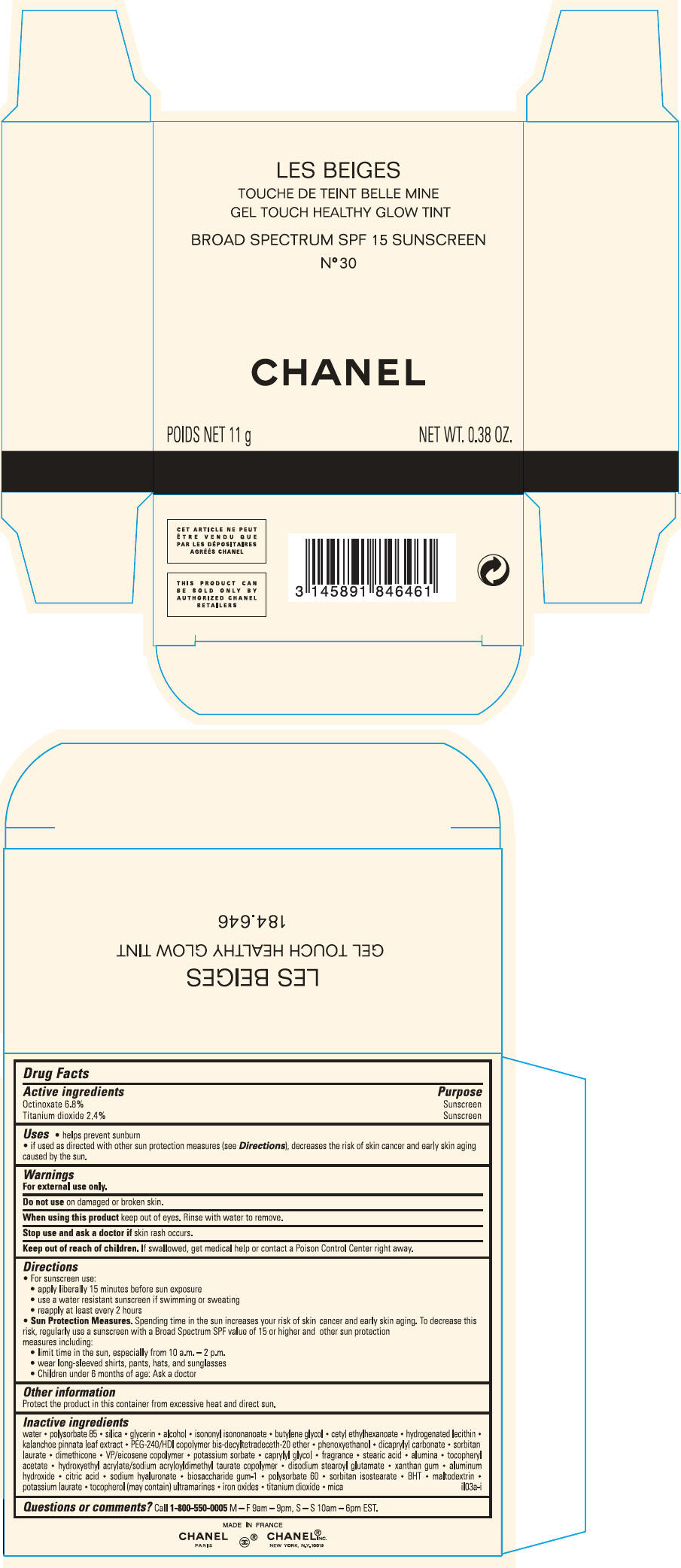
PRINCIPAL DISPLAY PANEL
LES BEIGES
GEL TOUCH HEALTHY GLOW TINT
BROAD SPECTRUM SPF 15 SUNSCREEN
N° 40
CHANEL
POIDS NET 11 g
NET WT. 0.38 OZ.
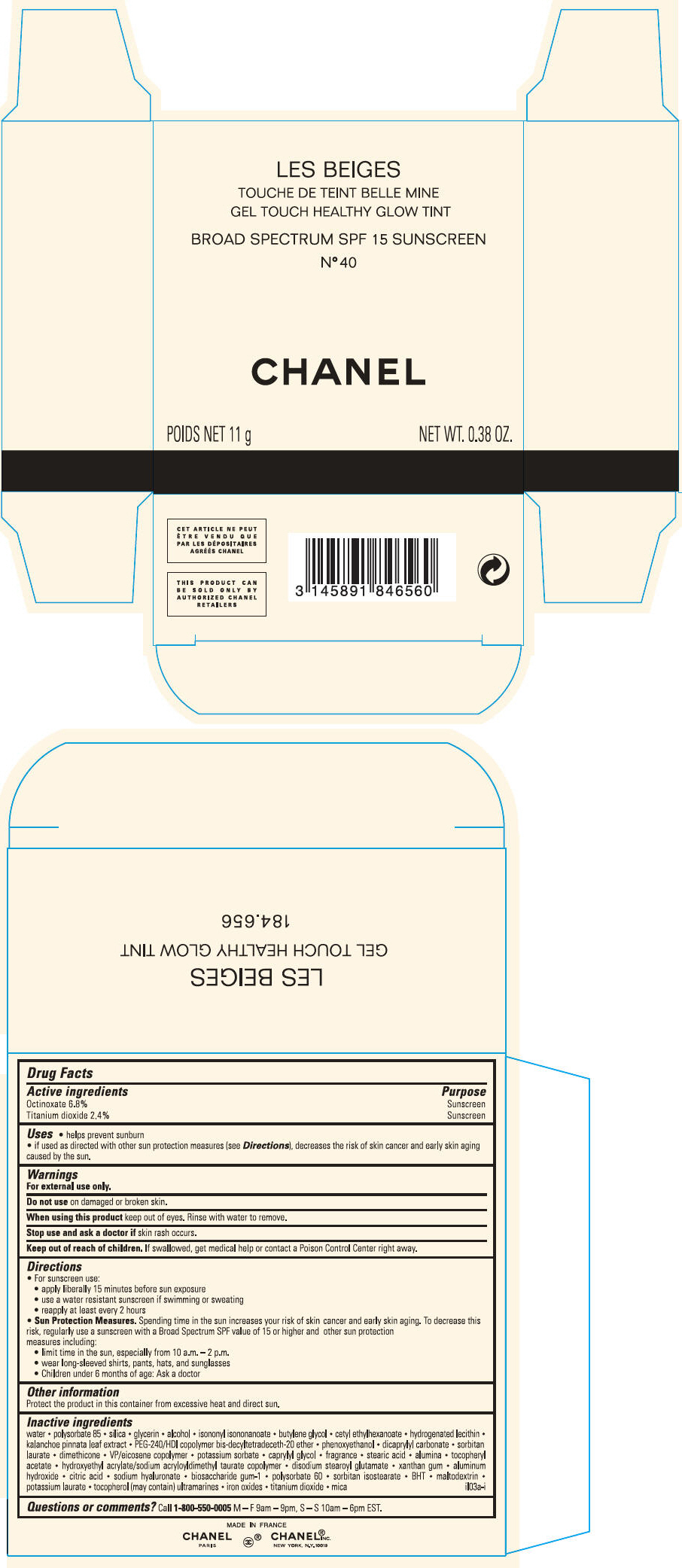
PRINCIPAL DISPLAY PANEL
LES BEIGES
GEL TOUCH HEALTHY GLOW TINT
BROAD SPECTRUM SPF 15 SUNSCREEN
N° 50
CHANEL
POIDS NET 11 g
NET WT. 0.38 OZ.
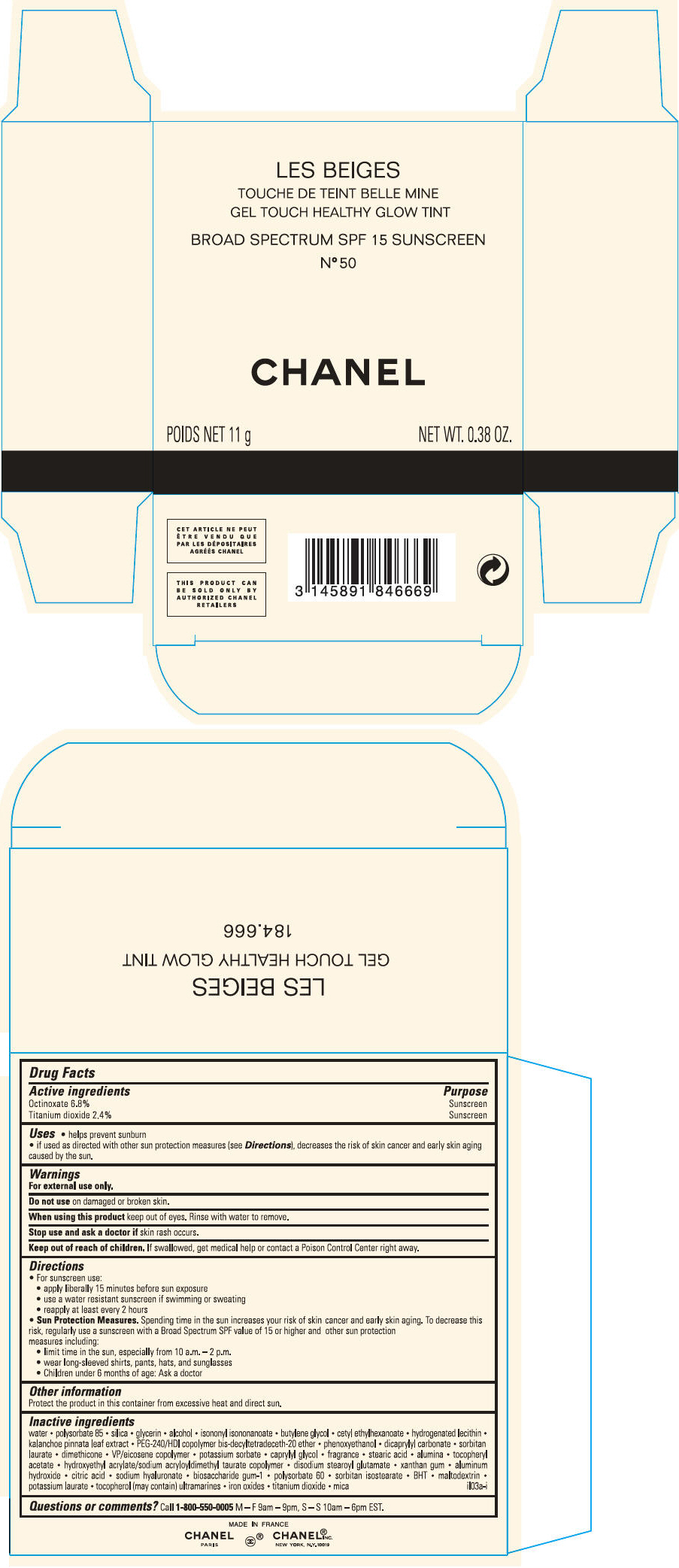
PRINCIPAL DISPLAY PANEL
LES BEIGES
GEL TOUCH HEALTHY GLOW TINT
BROAD SPECTRUM SPF 15 SUNSCREEN
N° 60
CHANEL
POIDS NET 11 g
NET WT. 0.38 OZ.
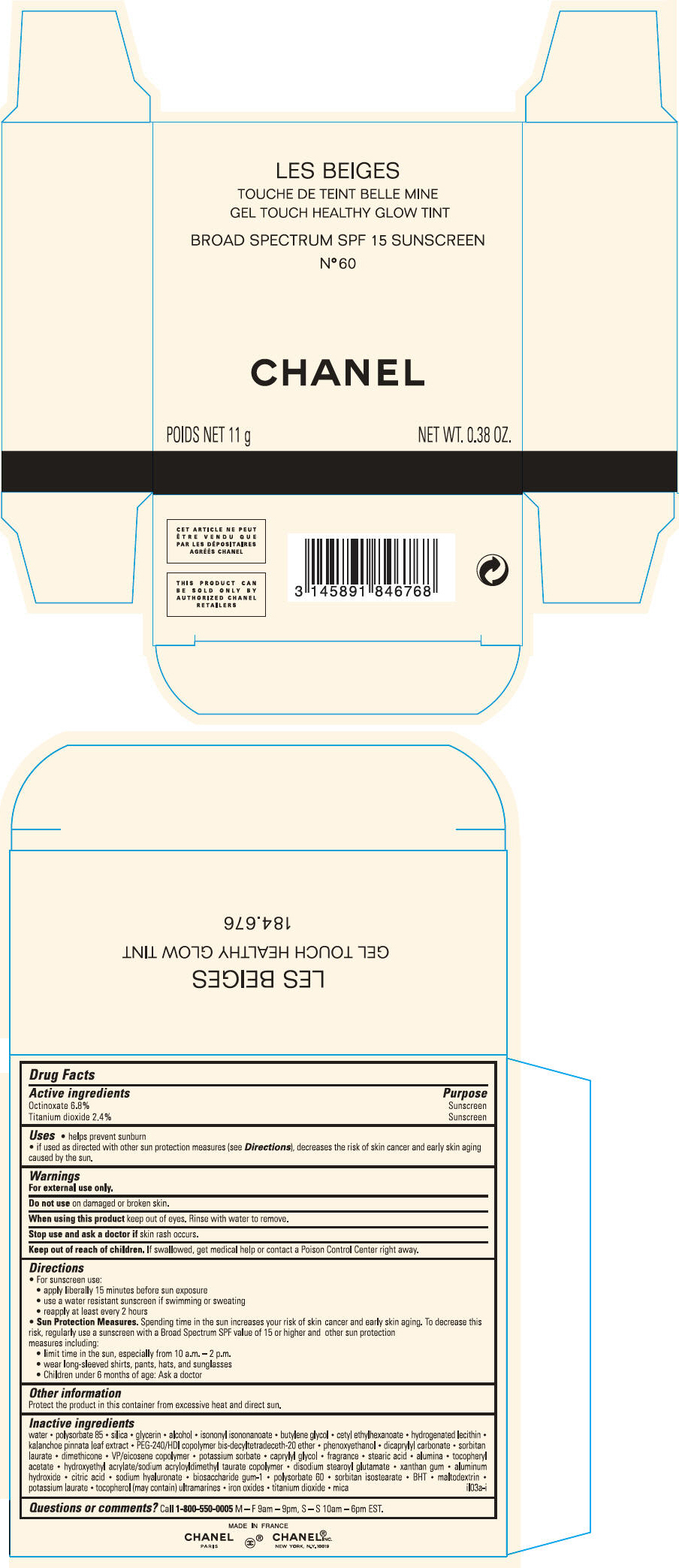
PRINCIPAL DISPLAY PANEL
LES BEIGES
GEL TOUCH HEALTHY GLOW TINT
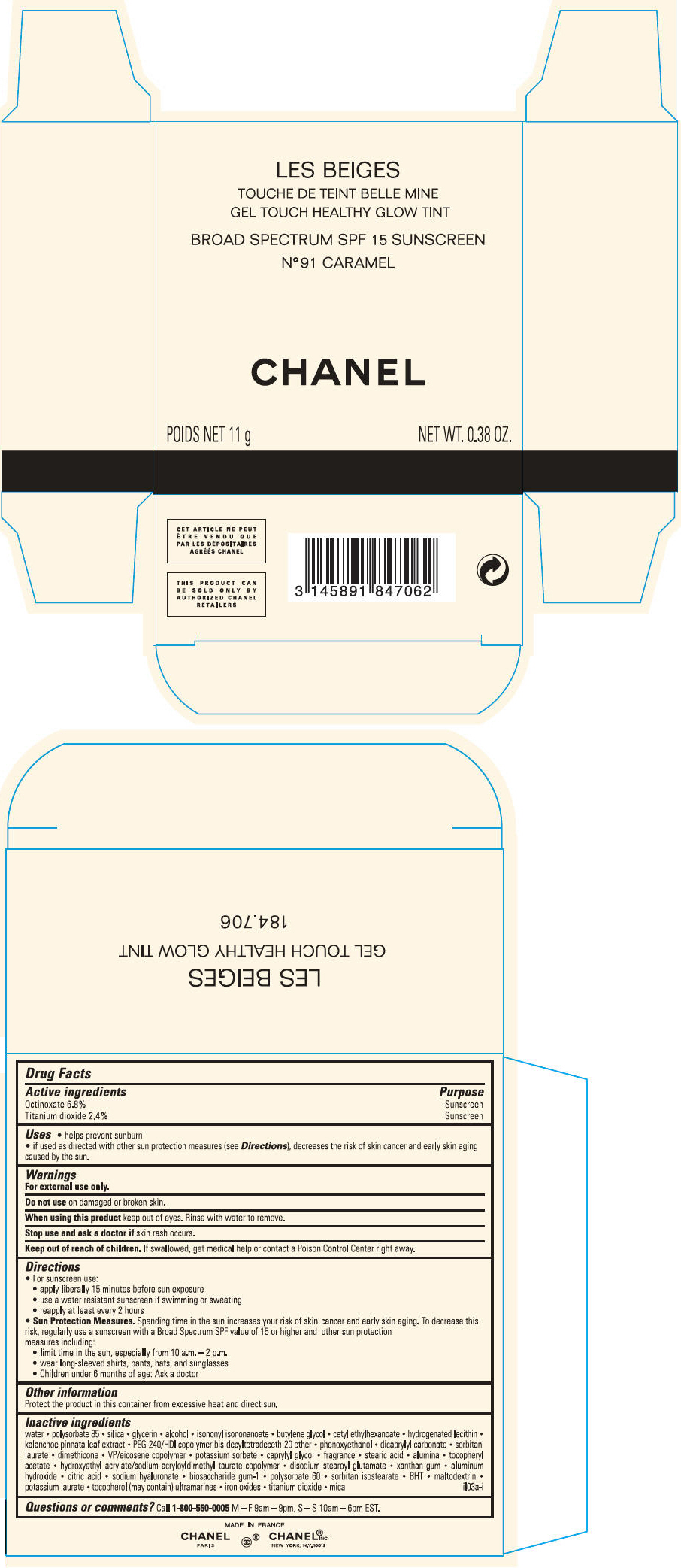
BROAD SPECTRUM SPF 15 SUNSCREEN
N° 91 CARAMEL
CHANEL
POIDS NET 11 g
NET WT. 0.38 OZ.