NDC Code(s) : 68180-947-02, 68180-947-01
Packager : Lupin Pharmaceuticals, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Betamethasone dipropionateBetamethasone dipropionate OINTMENT, AUGMENTED | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| LABELER - Lupin Pharmaceuticals, Inc.(089153071) |
| REGISTRANT - LUPIN LIMITED(675923163) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| LUPIN LIMITED | 650595213 | MANUFACTURE(68180-947), PACK(68180-947) | |
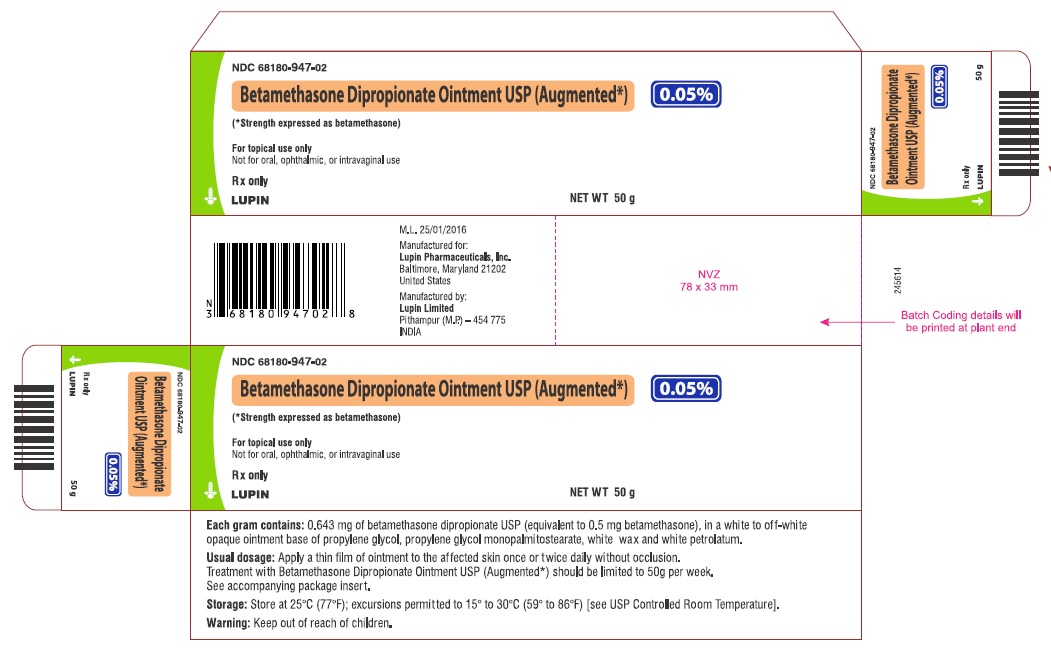
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 50 g Carton
NDC 68180-947-02
Betamethasone Dipropionate Ointment USP (Augmented*), 0.05%
(*Strength expressed as betamethasone)
For topical use only
Not for oral, ophthalmic, or intravaginal use
Rx only
Keep out of reach of children.
NET WT 50 g
PRINCIPAL DISPLAY PANEL - 50 g Container (tube) label
NDC 68180-947-02
Betamethasone Dipropionate Ointment USP (Augmented*), 0.05%
(*Strength expressed as betamethasone)
For topical use only
Not for oral, ophthalmic, or intravaginal use
Rx only
Keep out of reach of children.
NET WT 50 g









