NDC Code(s) : 65862-751-50, 65862-751-01, 65862-752-50, 65862-752-75
Packager : Aurobindo Pharma Limited
Category : Human Prescription Drug Label
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Cefixime cefixime POWDER, FOR SUSPENSION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Cefixime cefixime POWDER, FOR SUSPENSION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LABELER - Aurobindo Pharma Limited(650082092) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Aurobindo Pharma Limited | 918917639 | ANALYSIS(65862-751, 65862-752), MANUFACTURE(65862-751, 65862-752) | |
PRINCIPAL DISPLAY PANEL
NDC 65862-751-50
Rx only
Cefixime for
Oral Suspension, USP
100 mg/5 mL
when reconstituted
FOR ORAL USE ONLY
SHAKE WELL BEFORE USING
AUROBINDO 50 mL
(when reconstituted)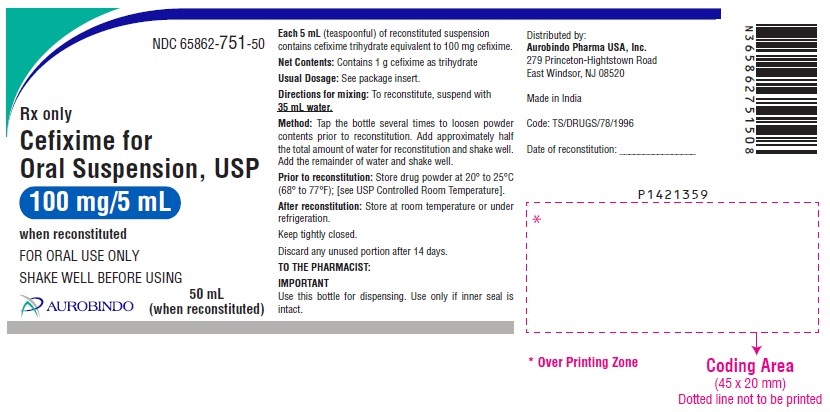
PRINCIPAL DISPLAY PANEL
NDC 65862-752-50
Rx only
Cefixime for
Oral Suspension, USP
200 mg/5 mL
when reconstituted
FOR ORAL USE ONLY
SHAKE WELL BEFORE USING
AUROBINDO 50 mL
(when reconstituted)








